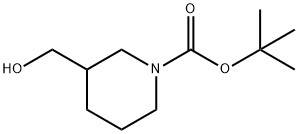
N-Boc-piperidine-3-methanol synthesis
- Product Name:N-Boc-piperidine-3-methanol
- CAS Number:116574-71-1
- Molecular formula:C11H21NO3
- Molecular Weight:215.29
4606-65-9

24424-99-5

116574-71-1
(1) Preparation of 3-(hydroxymethyl)-1-(tert-butoxycarbonyl)piperidine: To a solution of 3-hydroxymethylpiperidine (1.15 g, 10 mmol) in tetrahydrofuran (15 ml) was added triethylamine (2.8 ml, 20 mmol) at room temperature. Subsequently, a solution of di-tert-butyl dicarbonate (2.62 g, 10 mmol) in tetrahydrofuran (5 ml) was added slowly and dropwise with stirring. The reaction mixture was stirred continuously for 20 hours at room temperature. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was dissolved in ethyl acetate (50 ml), and the organic phase was washed sequentially with water and saturated saline, and then dried with anhydrous sodium sulfate. Finally, the solvent was removed by reduced pressure distillation to afford the target product tert-butyl 3-(hydroxymethyl)piperidine-1-carboxylate (2.15 g, 100% yield) as colorless crystals. The product was confirmed by 1H-NMR (CDCl3) and IR (KBr) characterization.1H-NMR (CDCl3) δ: 1.2-1.4 (2H, m), 1.46 (9H, s), 1.5-1.9 (4H, m), 2.8-3.3 (2H, m), 3.51 (2H, t, J = 6.10 Hz), 3.6-3.9 (2H, m) IR (KBr) cm-1: 3491, 1742, 1674, 1428, 1269, 1177, 1153, 858, 769.

4606-65-9
249 suppliers
$9.00/1g

24424-99-5
858 suppliers
$13.50/25G

121-44-8
895 suppliers
$9.00/5g

116574-71-1
284 suppliers
$8.00/5g
Yield:116574-71-1 96%
Reaction Conditions:
in dichloromethane
Steps:
31.A A.
A. N-Boc-3-hydroxymethylpiperidine To a stirred solution of 3-hydroxymethylpiperidine (15.1 g, 131 mmol) and Et3 N (21.9 mL, 158 mmol) in 100 mL of dichloromethane was added dropwise a solution of di-t-butyl dicarbonate (31.5 g, 144 mmol) in 100 mL of dichloromethane over 1 h. The reaction was stirred at room temperature for 18 h and then diluted with 200 mL of dichloromethane. The resulting solution was washed with 1N HCl solution (3*100 mL), saturated NaHCO3 solution (2*100 mL) and brine (1*100 mL). The organic layer was dried (MgSO4), filtered and concentrated in vacuo to give N-Boc-3-hydroxymethylpiperidine (27.0 g, 96%).
References:
Bristol-Myers Squibb Company US5583146, 1996, A

4606-65-9
249 suppliers
$9.00/1g

24424-99-5
858 suppliers
$13.50/25G

116574-71-1
284 suppliers
$8.00/5g
148763-41-1
155 suppliers
$8.00/5g

116574-71-1
284 suppliers
$8.00/5g
![tert-butyl 3-[(phenylamino)methyl]piperidine-1-carboxylate](/CAS/20200401/GIF/309735-44-2.gif)
309735-44-2
1 suppliers
inquiry

24424-99-5
858 suppliers
$13.50/25G

68-22-4
448 suppliers
$25.00/250mg

116574-71-1
284 suppliers
$8.00/5g

24424-99-5
858 suppliers
$13.50/25G

116574-71-1
284 suppliers
$8.00/5g