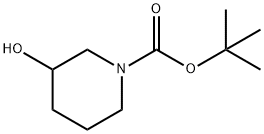
1-Boc-3-hydroxypiperidine synthesis
- Product Name:1-Boc-3-hydroxypiperidine
- CAS Number:85275-45-2
- Molecular formula:C10H19NO3
- Molecular Weight:201.26
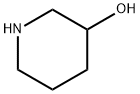
6859-99-0

24424-99-5

85275-45-2
1) At room temperature, 3-hydroxypiperidine (5.00 g) was dissolved in methanol (50 mL), to which a methanol (50 mL) solution of triethylamine (15.2 mL) and di-tert-butyl dicarbonate (11.9 g) were sequentially added. The reaction mixture was stirred for 15 hours. After completion of the reaction, the solvent was removed by evaporation under reduced pressure and the resulting residue was purified by silica gel column chromatography (eluent: ethyl acetate-chloroform) to afford tert-butyl 3-hydroxypiperidine-1-carboxylate as a white solid (9.86 g, 99% yield). The product was characterized by 1H-NMR (400 MHz, CDCl3) and EI-MS: 1H-NMR δ: 1.36-1.55 (2H, m), 1.45 (9H, s), 1.71-1.78 (1H, m), 1.88 (1H, m), 3.02-3.13 (2H, m), 3.52 (1H, m), 3.72-3.76 (2H, m); EI-MS m/z: 201 (M+).

6859-99-0
407 suppliers
$6.00/5g

24424-99-5
857 suppliers
$13.50/25G

85275-45-2
369 suppliers
$7.00/5g
Yield:85275-45-2 99%
Reaction Conditions:
with triethylamine in methanol at 20; for 15 h;
Steps:
34.1 1)
1) 3-Hydroxypiperidine-1-carboxylic acid tert-butyl ester Triethylamine (15.2 mL) and a solution of di-tert-butoxydicarbonate (11.9 g) in methanol (50 mL) were added to a solution of 3-hydroxypiperidine (5.00 g) in methanol (50 mL) at room temperature, and the mixture was stirred for 15 hours. The solvent of the reaction mixture was evaporated under reduced pressure, and the residue was purified through silica gel column chromatography (ethyl acetate - chloroform), to thereby give 3-hydroxypiperidine-1-carboxylic acid tert-butyl ester as a solid product (9.86 g, 99%). 1H-NMR(400MHz,CDCl3)δ:1.36-1.55(2H,m), 1.45(9H,s), 1.71-1.78(1H,m), 1.88(1H,m), 3.02-3.13(2H,m), 3.52(1H,m), 3.72-3.76(2H,m). EI-MS m/z:201 (M+).
References:
DAIICHI PHARMACEUTICAL CO., LTD. EP1762568, 2007, A1 Location in patent:Page/Page column 49-50

24424-99-5
857 suppliers
$13.50/25G

64051-79-2
186 suppliers
$9.00/1g

85275-45-2
369 suppliers
$7.00/5g
98977-36-7
413 suppliers
$6.00/1g

85275-45-2
369 suppliers
$7.00/5g

6859-99-0
407 suppliers
$6.00/5g
34619-03-9
29 suppliers
inquiry
811-93-8
215 suppliers
$10.00/250mg

85275-45-2
369 suppliers
$7.00/5g