
TERT-BUTYL 4-(AMINOCARBONYL)TETRAHYDROPYRIDINE-1(2H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-(AMINOCARBONYL)TETRAHYDROPYRIDINE-1(2H)-CARBOXYLATE
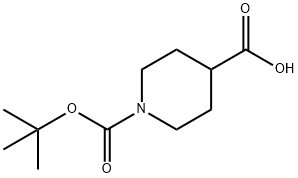
- CAS Number:91419-48-6
- Molecular formula:C11H20N2O3
- Molecular Weight:228.29
84358-13-4

91419-48-6
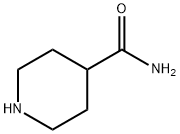
General procedure for the synthesis of tert-butyl 4-carbamoylpiperidine-1-carboxylate from 1-Boc-4-piperidinecarboxylic acid: a mixture of 1-(tert-butoxycarbonyl)piperidine-4-carboxylic acid (220 mg, 2.88 mmol), ammonium chloride (154 mg, 2.88 mmol), EDCI (367 mg, 1.92 mmol) and HOAT (195 mg. 1.44 mmol) were dissolved in dichloromethane (10 mL) at 0 °C, followed by dropwise addition of DIPEA (1.0 mL, 5.77 mmol). After dropwise addition, the reaction mixture was stirred at room temperature for 10 hours. After completion of the reaction, the reaction mixture was washed with distilled water (10 mL x 3). The organic layer was separated and dried with anhydrous Na2SO4 and subsequently concentrated under reduced pressure. The residue was purified by silica gel column chromatography using ethyl acetate as eluent to afford tert-butyl 4-carbamoylpiperidine-1-carboxylate as a white solid (160 mg, 73% yield).1H NMR (400 MHz, CD3OD): δ 4.11 (d, J = 13.4 Hz, 2H), 2.76-2.86 (m, 2H), 2.38-2.45 ( m, 1H), 1.79 (d, J = 11.2 Hz, 2H), 1.52-1.62 (m, 2H), 1.47 (s, 9H).MS-ESI: m/z 173.20 [M-55]+.
39546-32-2
350 suppliers
$6.00/5g

24424-99-5
865 suppliers
$13.50/25G

91419-48-6
226 suppliers
$20.80/1G
Yield:91419-48-6 95%
Reaction Conditions:
with 4-methylmorpholine N-oxide in 1,4-dioxane;Product distribution / selectivity;
Steps:
1
The ring nitrogen atom of isonipecotamide 1 (commercially available from Aldrich) was Boc-protected in 95% isolated yield by using Boc20 in dioxane and NMO as the base.
References:
WO2004/2483,2004,A1 Location in patent:Page/Page column 21-22

84358-13-4
382 suppliers
$5.00/5g

91419-48-6
226 suppliers
$20.80/1G

124443-68-1
254 suppliers
$6.00/5g

91419-48-6
226 suppliers
$20.80/1G
![1-PIPERIDINECARBOXYLIC ACID,4-[(HYDROXYIMINO)METHYL]-,1,1-DIMETHYLETHYL ESTER](/CAS/GIF/190446-85-6.gif)
190446-85-6
32 suppliers
$60.00/100mg

91419-48-6
226 suppliers
$20.80/1G

24424-99-5
865 suppliers
$13.50/25G

91419-48-6
226 suppliers
$20.80/1G