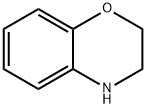
Benzomorpholine synthesis
- Product Name:Benzomorpholine
- CAS Number:5735-53-5
- Molecular formula:C8H9NO
- Molecular Weight:135.16

106-93-4
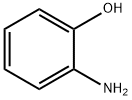
95-55-6

5735-53-5
To a suspension of 2-aminophenol (1.0 g, 9.2 mmol) and potassium carbonate (6.36 g, 46 mmol) in anhydrous DMF (10 mL) was added 1,2-dibromoethane (2.59 g, 13.8 mmol). The reaction mixture was heated at 125 °C with stirring for 15 hours. After completion of the reaction, it was cooled to room temperature, the mixture was poured into crushed ice and extracted with ethyl acetate (3 × 20 mL). The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The resulting crude product was purified by silica gel column chromatography with ethyl acetate/petroleum ether (1:10, v/v) as eluent to afford 3,4-dihydro-2H-benzo[b][1,4]oxazine as a red oil (0.81 g, 65% yield). The product was analyzed by LC-MS (ES-API): retention time 7.51 min; calculated value C8H9NO [M+H]+ 136.1, measured value 136.1.

31507-30-9
9 suppliers
inquiry

5735-53-5
143 suppliers
$39.00/1G
Yield: 88%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;toluene-4-sulfonic acid in ethanol at 50; under 37503.8 Torr; for 2 h;
Steps:
1.ii
In step (ii), intermediate c was reduced and cyclised to give the following fuel additive e Specifically, 2-(2-nitrophenoxy)acetonitrile (2.0 g, 11 mmol), //TSA (50 mg), 10 % Pd/C (100 mg) were stirred in ethanol (35 ml) under 50 bar at 50 °C for 2 hours. The reaction mixture was filtered, the solvent was evaporated, the residue was taken up into diethyl ether and filtered to remove solids. The solvent was removed in vacuo to give 1.34 g (88 % yield) of fuel additive e as a clear brown oil.
References:
BP OIL INTERNATIONAL LIMITED;FILIP, Sorin, Vasile WO2019/129591, 2019, A1 Location in patent:Page/Page column 33

102236-25-9
16 suppliers
inquiry

5735-53-5
143 suppliers
$39.00/1G
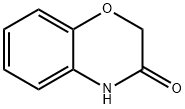
5466-88-6
193 suppliers
$25.00/5g

5735-53-5
143 suppliers
$39.00/1G

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)