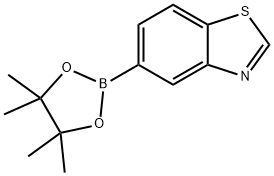
5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazole synthesis
- Product Name:5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazole
- CAS Number:1073354-91-2
- Molecular formula:C13H16BNO2S
- Molecular Weight:261.15

768-11-6

73183-34-3
![5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazole](/CAS/GIF/1073354-91-2.gif)
1073354-91-2
A mixture was prepared from 5-bromobenzo[d]thiazole (428 mg, 2.00 mmol), bis(pinacolato)diboron (558 mg, 2.20 mmol) and potassium acetate (588 mg, 6.00 mmol) in dimethylsulfoxide (7 mL). The mixture was stirred under nitrogen protection for 5 min. Subsequently, [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride dichloromethane complex (293 mg, 0.40 mmol) was added and the reaction mixture was stirred for 3 hours at 85 °C. Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad and the filter cake was washed with ethyl acetate (75 mL) followed by water (20 mL). The filtrate was diluted with ethyl acetate (100 mL) and washed sequentially with water (2 x 75 mL) and brine (75 mL). The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel as stationary phase, eluent was a mixture of dichloromethane and ethyl acetate, gradient elution, volume fraction of ethyl acetate was increased from 0% to 50%) to afford the target product 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazole (200 mg, 38% yield) as a light brown solid. The structure of the product was confirmed by 1H NMR (300 MHz, DMSO-d6) and ESI MS: 1H NMR δ 9.41 (s, 1H), 8.32 (s, 1H), 8.18 (d, 1H, J = 8.0 Hz), 7.72 (d, 1H, J = 8.0 Hz), 1.33 (s, 12H); ESI MS m/z 262.1 [M + H ]+.

768-11-6
129 suppliers
$10.00/1g

73183-34-3
589 suppliers
$6.00/5g
![5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazole](/CAS/GIF/1073354-91-2.gif)
1073354-91-2
70 suppliers
$49.00/100mg
Yield:1073354-91-2 59%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 100; for 16 h;Inert atmosphere;
References:
WO2022/63140,2022,A1 Location in patent:Paragraph 0139-0141
2786-51-8
73 suppliers
$40.00/1g

73183-34-3
589 suppliers
$6.00/5g
![5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazole](/CAS/GIF/1073354-91-2.gif)
1073354-91-2
70 suppliers
$49.00/100mg