Levofloxacin synthesis
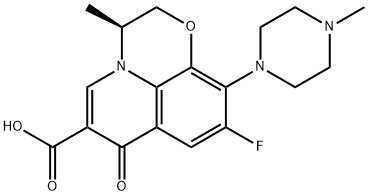
- Product Name:Levofloxacin
- CAS Number:100986-85-4
- Molecular formula:C18H20FN3O4
- Molecular Weight:361.37
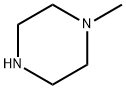
109-01-3
668 suppliers
$5.00/5G

100986-89-8
345 suppliers
$8.00/250mg

100986-85-4
667 suppliers
$5.00/100mg
Yield:100986-85-4 97.1%
Reaction Conditions:
with potassium hydroxide in water at 55; for 24 h;Reagent/catalyst;Time;Temperature;Solvent;
Steps:
4
,3-dihydro-3-methyl-7-oxo-7H-pyrido [1,2,3-Δ] - [1,4] Benzoxazine-6-carboxylate, 4.5 g of water, 4.5 g of N-methylpiperazine and 0.22 g (81%) of potassium hydroxide were added and the reaction was incubated at 55 ° C and the alcohol , And the reaction was allowed to proceed for about 6 hours.The reaction was warmed to reflux for about 18 hours.N-methylpiperazine was replaced by N-methylpiperazine.The residue of levofloxacin crude, dissolved with chloroform and water, after acid and alkali pH adjustment, extraction, washing, concentration and other steps.(Methanol: dichloromethane = 1: 8), and the solid was combined with the cold methanol rinse to obtain the finished product of levofloxacin Yield: 97.1%.The compound was confirmed to be levofloxacin product by the same method as the product obtained in Example 1, by measuring the melting point, determining the molecular weight by high resolution mass spectrometry, and confirming the product by NMR.
References:
CN103755722,2016,B Location in patent:Paragraph 0052-0053

109-01-3
668 suppliers
$5.00/5G
![Ethyl (S)-9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate](/CAS/GIF/106939-34-8.gif)
106939-34-8
233 suppliers
$12.00/5g

100986-85-4
667 suppliers
$5.00/100mg
50-00-0
894 suppliers
$10.00/25g
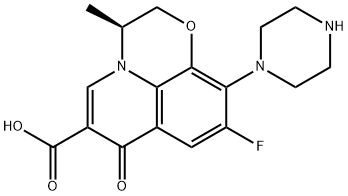
117707-40-1
101 suppliers
$86.00/5mg

100986-85-4
667 suppliers
$5.00/100mg
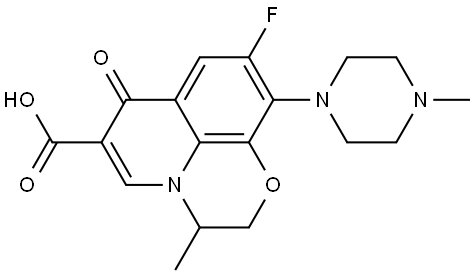
82419-36-1
501 suppliers
$5.00/250mg

100986-85-4
667 suppliers
$5.00/100mg
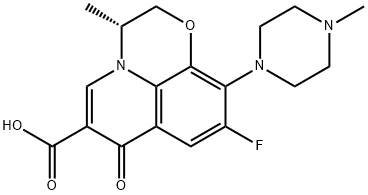
100986-86-5
110 suppliers
$60.00/1mg

1036016-10-0
24 suppliers
inquiry

100986-85-4
667 suppliers
$5.00/100mg