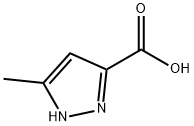
5-Methyl-1H-pyrazole-3-carboxylic acid synthesis
- Product Name:5-Methyl-1H-pyrazole-3-carboxylic acid
- CAS Number:402-61-9
- Molecular formula:C5H6N2O2
- Molecular Weight:126.11

67-51-6

3112-31-0

402-61-9
General procedure for the synthesis of 3,5-pyrazoledicarboxylic acid and 5-methyl-1H-pyrazole-3-carboxylic acid from 3,5-dimethylpyrazole: 3,5-dimethyl-1H-pyrazole (78.5 g, 0.818 mol) was dissolved in 700 mL of water heated to 70 °C, potassium permanganate (517 g, 3.271 mol) was added slowly, and the temperature was kept no higher than 90 °C. After completion of the reaction, the mixture was cooled to room temperature, filtered to remove the resulting MnO2 precipitate, and washed with distilled water. The filtrate was acidified to pH 2 with dilute aqueous hydrochloric acid and allowed to stand overnight. The precipitate was collected by filtration and washed with distilled water to afford 3,5-pyrazoledicarboxylic acid (41.75 g, 33% yield) as white crystals with melting point 257-258 °C. 1H NMR (D2O, δ): 7.07 (s, 1H, 4-H). After isolation of 3,5-pyrazoledicarboxylic acid, the remaining aqueous phase filtrate was neutralized to pH 5-6, the precipitate was collected by filtration and washed with distilled water to afford 5-methyl-1H-pyrazole-3-carboxylic acid (18.1 g, 18% yield) as white crystals, melting point 210-211°C.1H NMR (D2O, δ): 2.25 (s, 3H, CH3), 6.42 (s, 1H, 4-H). 4-H).
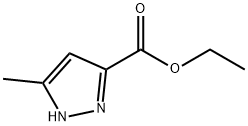
4027-57-0
255 suppliers
$6.00/1g

402-61-9
209 suppliers
$10.00/500mg
Yield: 88%
Reaction Conditions:
with sodium hydroxide;ethanol for 1 h;Heating / reflux;
Steps:
ii.b
To a solution of 5-methylpyrazole-3-carboxylic acid ethyl ester (4.94 g, 32.0 mmol) in abs. EtOH (80 mL) was added NaOH (6.4 g, 160 mmol). The mixture was heated at reflux for 1 h and cooled to 20 C. The mixture was acidified with HC1 (aq., 2 M, 85 mL, 170 mmol) and the pH adjusted to 3 withNaOH (aq., 2 M). The mixture was extracted with EtOAc (200 mL). The organic phase was washed with NaCI (aq, sat, 50 mL) and concentrated to give the title compound (3.54 g, 88 %) as a white solid. 5 ^ NMR (DMSO-ds, 400 MHz) S 12.83 (br. s, 1H). 6.43 (s, 1H), 2.22 (s, 3H).
References:
BIOLIPOX AB WO2006/32851, 2006, A1 Location in patent:Page/Page column 39-40
10010-93-2
225 suppliers
$6.00/100mg

402-61-9
209 suppliers
$10.00/500mg

67-51-6
572 suppliers
$6.00/25g

3112-31-0
216 suppliers
$18.00/1g

402-61-9
209 suppliers
$10.00/500mg

67-51-6
572 suppliers
$6.00/25g

402-61-9
209 suppliers
$10.00/500mg
40535-14-6
105 suppliers
inquiry

402-61-9
209 suppliers
$10.00/500mg