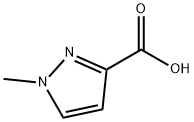
1-Methyl-1H-pyrazole-3-carboxylic acid synthesis
- Product Name:1-Methyl-1H-pyrazole-3-carboxylic acid
- CAS Number:25016-20-0
- Molecular formula:C5H6N2O2
- Molecular Weight:126.11

1621-91-6

77-78-1
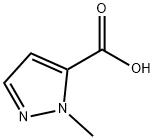
16034-46-1

25016-20-0
Over 45 minutes, dimethyl sulfate (236 g, 177 mL, 1.87 mol) was slowly added dropwise to a stirred solution of pyrazole-3-carboxylic acid (200 g, 1.78 mol) dissolved in 20% aqueous sodium hydroxide (850 mL) at 40 °C. The reaction mixture was heated at 80 °C for 2 h and subsequently cooled to room temperature. The reaction mixture was filtered and the filtrate was acidified to pH 1 with concentrated hydrochloric acid, the precipitate was collected by filtration, washed with water and dried under vacuum to afford 1-methyl-1H-pyrazole-5-carboxylic acid (85 g, 38% yield). The remaining filtrate was concentrated to 800 mL in vacuum and extracted with chloroform (15 x 400 mL) several times. The organic phases were combined, dried with anhydrous magnesium sulfate and concentrated in vacuum to remove the solvent. The residue was recrystallized by isopropanol to give 1-methyl-1H-pyrazole-3-carboxylic acid (74 g) as a white crystalline solid.
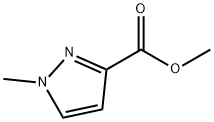
17827-61-1
110 suppliers
$17.00/250mg

25016-20-0
209 suppliers
$9.00/100mg
Yield:25016-20-0 98%
Reaction Conditions:
with lithium hydrochloride monohydrate in tetrahydrofuran;methanol;water at 0 - 30; for 3 h;
Steps:
99.2
Step 2: To a solution of step 1 intermediate (7.0 g, 50 mmol) in a mixture of THF (20 mL) and MeOH (20 mL) was added a solution of LiOH H20 (4.2 g, 100 mmol) in water (20 mL) at 0°C. The RM was stirred at rt for 3 h. The RM was concentrated and subsequently diluted with water. The aqueous layer was acidified with sat. NaHS04 solution up to pH~4-5 and extracted with EtOAc. The combined organic layers were dried and concentrated under reduced pressure to yield the desired product (6.2 g, 98%). LC-MS (Method 3): m/z [M+H]+ = 127.2 (MW calc. 126.11); R, = 0.44 min.
References:
WO2015/22073,2015,A1 Location in patent:Page/Page column 90; 91

1621-91-6
307 suppliers
$8.00/5g

77-78-1
326 suppliers
$22.00/25g

16034-46-1
248 suppliers
$6.00/1g

25016-20-0
209 suppliers
$9.00/100mg
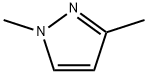
694-48-4
228 suppliers
$6.00/1g

25016-20-0
209 suppliers
$9.00/100mg

1621-91-6
307 suppliers
$8.00/5g

77-78-1
326 suppliers
$22.00/25g

16034-46-1
248 suppliers
$6.00/1g

25016-20-0
209 suppliers
$9.00/100mg