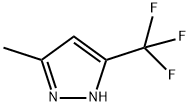
3-METHYL-5-(TRIFLUOROMETHYL)PYRAZOLE synthesis
- Product Name:3-METHYL-5-(TRIFLUOROMETHYL)PYRAZOLE
- CAS Number:10010-93-2
- Molecular formula:C5H5F3N2
- Molecular Weight:150.1
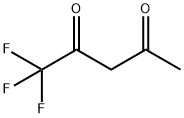
367-57-7

10010-93-2
Example 127: Preparation of 5-methyl-3-trifluoromethyl-1H-pyrazole Hydrazine hydrate (945 μl, 30 mmol) was slowly added to a solution of 1,1,1-trifluoro-2,4-pentanedione (4.62 g, 30 mmol) in methanol (20 ml) at 0 °C. The reaction mixture was gradually warmed up to room temperature and stirred at that temperature for 16 hours. After completion of the reaction, the solvent was removed by concentration under reduced pressure to give 5-methyl-3-trifluoromethyl-1H-pyrazole as a yellow solid (4.5 g, 95% yield). 1H-NMR (400 MHz, CDCl3): δ 2.35 (s, 3H, CH3), 6.32 (s, 1H, CH), 9.88 (bs, 1H, NH) ppm.

367-57-7
292 suppliers
$10.00/2 g

10010-93-2
225 suppliers
$6.00/100mg
Yield:10010-93-2 95%
Reaction Conditions:
with hydrazine in methanol at 0 - 20; for 16 h;
Steps:
2.I27
Example 127: Preparation of 5-methyl-3-trifluoromethyl-l/jr-pyrazoleTo a solution of trifluoroacetyl acetone (4.62 g, 30 mmol) in methanol (20 ml), was added slowly at O0C a solution of hydrazine (945 μl, 30 mmol). The reaction mixture was allowed to warm to room temperature and stirred at room temperature for 16 hours.
References:
SYNGENTA LIMITED WO2007/71900, 2007, A1 Location in patent:Page/Page column 88-89

367-57-7
292 suppliers
$10.00/2 g

10010-93-2
225 suppliers
$6.00/100mg

10010-93-2
225 suppliers
$6.00/100mg