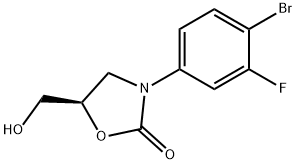
(5R)-3-(4-BROMO-3-FLUOROPHENYL)-5-HYDROXYMETHYLOXAZOLIDIN-2-ONE synthesis
- Product Name:(5R)-3-(4-BROMO-3-FLUOROPHENYL)-5-HYDROXYMETHYLOXAZOLIDIN-2-ONE
- CAS Number:444335-16-4
- Molecular formula:C10H9BrFNO3
- Molecular Weight:290.09

510729-01-8
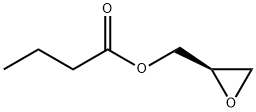
60456-26-0

444335-16-4
General procedure for the synthesis of (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethoxazolidin-2-one from phenylmethyl N-(4-bromo-3-fluorophenyl)formate and glycidyl butyrate: 119 g (367 mmol) of phenylmethyl N-(4-bromo-3-fluorophenyl)formate was dissolved in 300 mL of tetrahydrofuran and 150 mL of dimethylformamide mixed solvent and 38.19 g (477 mmol) of lithium tert-butoxide was added slowly dropwise at 0 °C. The reaction solution was stirred for 10 min, then 63 mL (440 mmol) of (R)-glycidyl butyrate and 21 mL (550 mmol) of methanol were added, and the resulting solution continued to be stirred for 3 h at room temperature. Upon completion of the reaction, the pH of the reaction mixture was adjusted to about 6 with aqueous ammonium chloride, followed by concentration under reduced pressure. The concentrate was dissolved in 1000 mL of 80% ethyl acetate/hexane mixed solvent, washed sequentially with water and saturated aqueous sodium chloride solution (brine), and the organic phase was dried over anhydrous sodium sulfate. After removal of the solvent under reduced pressure, purification by column chromatography afforded 93 g (320 mmol) of (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethoxazolidin-2-one as a white solid (yield: 87%).1H NMR (600 MHz, CDCl3) δ 7.53 (m, 2H), 7.15 (dd, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 4.77 (m, 1H), 4.00 (m, 3H), 3.77 (m, 1H), 2.10 (t, J = 6.0 Hz, 1H).

149524-42-5
98 suppliers
$34.00/100mg

444335-16-4
215 suppliers
$8.00/250mg
Yield:444335-16-4 92%
Reaction Conditions:
with silver trifluoroacetate;bromine chloride in acetonitrile at 20; for 24 h;
Steps:
2.3 (R) -3- (4-bromo-3-fluorophenyl) -2-oxo-5-oxazolidinylmethanol(Formula 5-a)
Will be 30 grams(R) -3- (3-fluorophenyl) -2-oxo-5-oxazolidinylmethanolDissolved in 300 ml of acetonitrile,And will be 46 gramsSilver trifluoroacetate(CF3COOAg) and 30.5 g of BrCl were added to the solution,After stirring at room temperature for 1 day, water was added to the solution and extracted with ethyl acetate. The resulting organic layer was separated and washed with brine and dehydrated. The residue was then filtered, concentrated in vacuo and dried, yielding 37.8 g of the title compound in 92% yield.
References:
CN106146559,2016,A Location in patent:Paragraph 0044; 0046

510729-01-8
162 suppliers
$7.00/100mg

60456-26-0
305 suppliers
$14.14/1gm:

444335-16-4
215 suppliers
$8.00/250mg
60827-45-4
271 suppliers
$14.00/5g

288570-67-2
0 suppliers
inquiry

444335-16-4
215 suppliers
$8.00/250mg
656-65-5
316 suppliers
$5.00/1g

444335-16-4
215 suppliers
$8.00/250mg

149524-47-0
54 suppliers
inquiry

444335-16-4
215 suppliers
$8.00/250mg