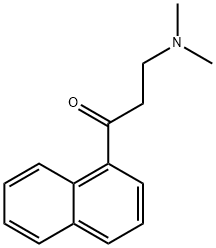
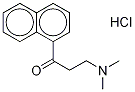
3-(dimethylamino)-1-(naphthalen-5-yl)propan-1-one synthesis
- Product Name:3-(dimethylamino)-1-(naphthalen-5-yl)propan-1-one
- CAS Number:10320-49-7
- Molecular formula:C15H17NO
- Molecular Weight:227.3
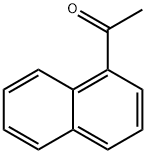
941-98-0
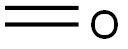
50-00-0
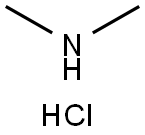
506-59-2

10320-49-7
1-Acetylnaphthalene (8 g, 0.047 mol), paraformaldehyde (2 g, 0.065 mol) and dimethylamine hydrochloride (5.3 g, 0.065 mol) were dissolved in ethanol (15 mL), followed by the addition of 0.6 mL of concentrated hydrochloric acid. The reaction mixture was heated to reflux for 12 hours, after which it was cooled to room temperature and refrigerated at -20 °C overnight. A white solid precipitated from the reaction mixture, which was separated by filtration and washed with ethanol. The resulting white solid was dissolved in water and 50% aqueous sodium carbonate solution (5.8 g, 0.54 mol) was slowly added. After stirring for 10-15 min, the aqueous phase was extracted with ethyl acetate (20 mL x 3), the organic layers were combined, washed sequentially with water (15 mL x 2) and saturated saline (15 mL x 2), and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure to obtain the target compound 3-dimethylamino-1-(naphthalen-5-yl)acetone as a colorless oil (8 g, 70% yield).

941-98-0
379 suppliers
$5.00/10g
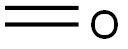
50-00-0
892 suppliers
$10.00/25g

506-59-2
568 suppliers
$5.00/5 g

10320-49-7
122 suppliers
$30.00/250mg
Yield: 89%
Reaction Conditions:
Stage #1:1'-naphthacetophenone;formaldehyd;N,N-dimethylammonium chloride in ethanol for 2 h;Reflux;Mannich Aminomethylation;
Stage #2: with sodium hydrogencarbonate in water
Steps:
3-(Dimethylamino)-1-(naphthalen-1-yl) propan-1-one (10a).
General procedure: In 500mL three-necked bottle, 1-(naphthalen-1-yl) ethanone (9a, 25g, 0.15mol), dimethylamine hydrochloride (15.6g, 0.19mol) and paraformaldehyde (7.2g, 0.08mol) were placed in the bottle. After the addition of 0.3mL of concentrated hydrochloric acid in 40mL of 95percent ethanol, the mixture was refluxed for 2h. Then the acetone (100mL) was added while the solution was still hot. Cooled slowly to room temperature and chilled overnight in the refrigerator. Filtered and washed with acetone (2×20mL) to afford white solid 3-(dimethylamino)-1-(naphthalen-1-yl)propan-1-one Hydrochloride (26.8g, 69percent). The solid was dissolved in water, alkalinized with saturated Sodium hydrogen carbonate aqueous solution and extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, then filtered and concentrated to get colorless oleosus product (10a, 20.5g, 89percent) which was easily decomposed but suitable for next step without further purification. Compounds 10b-10f were prepared without further purification from different commercially available substituted-ethanones in a range of 59–67percent yields.
References:
Cao, Ruiyuan;Fan, Shiyong;Li, Song;Liu, Ping;Lu, Yu;Wang, Bin;Wang, Xiaokui;Zhong, Wu [Bioorganic Chemistry,2020,vol. 102]
5409-58-5
200 suppliers
$15.00/5g

10320-49-7
122 suppliers
$30.00/250mg
654655-80-8
36 suppliers
$390.00/10 mg

654655-69-3
249 suppliers
$8.00/250mg

10320-49-7
122 suppliers
$30.00/250mg

941-98-0
379 suppliers
$5.00/10g
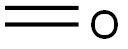
50-00-0
892 suppliers
$10.00/25g
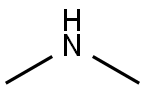
124-40-3
549 suppliers
$18.00/100ml

10320-49-7
122 suppliers
$30.00/250mg

941-98-0
379 suppliers
$5.00/10g

506-59-2
568 suppliers
$5.00/5 g

10320-49-7
122 suppliers
$30.00/250mg