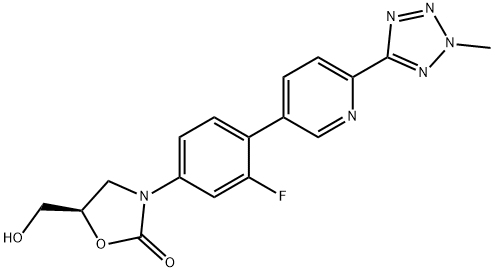
Torezolid synthesis
- Product Name:Torezolid
- CAS Number:856866-72-3
- Molecular formula:C17H15FN6O3
- Molecular Weight:370.34

1056039-83-8
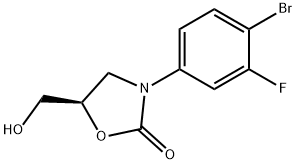
444335-16-4

856866-72-3
Under nitrogen protection, 1,4-dioxane (400 mL), 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (20.0 g, 83.3 mmol, 1.0 equiv), bis(pinacolato)diboron (25.3 g, 100 mmol, 1.2 equiv), potassium acetate (20.4 g, 208 mmol, 2.5 equivalent) and bis(triphenylphosphine)dichloropalladium (1.17 g, 1.4 mmol, 0.015 equivalent). The reaction mixture was heated to reflux and the progress of the reaction was monitored by TLC using 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine as reference. Upon completion of the reaction, it was cooled to below 20°C. Subsequently, (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethoxazolidin-2-one (21.7 g, 75.0 mmol, 0.9 equiv), potassium carbonate (34.5 g, 250 mmol, 3.0 equiv), palladium acetate (0.14 g, 0.62 mmol, 0.0075 equiv), and triphenylphosphine ( 0.65 g, 2.5 mmol, 0.03 eq.) and warmed to reflux under nitrogen protection. The progress of the reaction of 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine was tracked by TLC until completion. After completion of the reaction, it was cooled to room temperature and stirred for 16 hours. The reaction was filtered under reduced pressure and the filter cake was washed sequentially with water (200 mL) and methanol (200 mL). The filter cake was collected and air dried at 80°C to afford 25.8 g of the target product (R)-3-(3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-(hydroxymethyl)oxazolidin-2-one (Compound I) as an off-white powder in 84% yield.

444335-16-4
215 suppliers
$8.00/250mg

380380-64-3
261 suppliers
$10.00/250mg

856866-72-3
264 suppliers
inquiry
Yield:856866-72-3 83%
Reaction Conditions:
Stage #1:(R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxylmethyl)oxazolidin-2-one with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate;bis(pinacol)diborane in dimethyl sulfoxide at 80; for 14 h;Inert atmosphere;
Stage #2:5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;caesium carbonate in 1,4-dioxane;water at 70; for 3 h;Inert atmosphere;
Steps:
1 Example 1: Preparation of (R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (Formula V)
DMSO (100 ml) was added to a 250 ml reaction flask,(5R) -3- (4-bromo-3-fluorophenyl) -5-hydroxymethyloxazolidin-2-one(10 g, 34.5 mmol), pinacolate (17.52 g, 69 mmol),[1,1'-bis (diphenylphosphino) ferrocene] dichloropalladium dichloromethane complex (1.41 g, 1.73 mmol)And potassium acetate (13.5 g, 138 mmol), and the temperature was raised to 80 ° C under a nitrogen atmosphere,For 14 hours. The heating was stopped, the solution was cooled to room temperature, and extracted with water / ethyl acetate 3 times. The organic layers were combined and the organic layer was washed with saturated waterBrine, dried over anhydrous sodium sulfate and concentrated by suction filtration. The concentrated product of the above step was added to a 250 ml reaction flask,1,4-dioxane (100 ml) was added,5-bromo-2- (2-methyl-2H-tetrazol-5-yl) pyridine(8.28 g, 34.5 mmol),[1,1'-bis (diphenylphosphino) ferrocene] dichloropalladium dichloromethane complex (0.56 g, 0.69 mmol)And cesium carbonate aqueous solution (50 ml, containing 33.72 g of cesium carbonate, 103.5 mmol),Under nitrogen protection,The temperature was raised to 70 ° C,The reaction was carried out for 3 hours,Dichloromethane was added for extraction.The separated organic phase was washed with saturated brine,Anhydrous sodium sulfate dehydration,filter,Concentrated in vacuo and purified by column chromatography,10.6 g of a solid was obtained,The yield was 83.0%HPLC purity was 98.34% (area normalization method).
References:
Chia Tai Tianqing Pharmaceutical Group Co., Ltd.;Zhu, Yizhong;Zhang, Xiquan;Liu, Fei;Gu, Hongmei;Zhu, Bo;Tang, Song;Wang, Lulu CN105418678, 2016, A Location in patent:Paragraph 0070; 0071; 0072; 0073; 0074; 0075

1056039-83-8
125 suppliers
$16.00/250mg

444335-16-4
215 suppliers
$8.00/250mg

856866-72-3
264 suppliers
inquiry
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
135 suppliers
inquiry
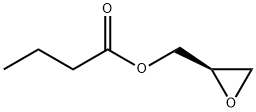
60456-26-0
305 suppliers
$14.14/1gm:

856866-72-3
264 suppliers
inquiry
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
135 suppliers
inquiry

856866-72-3
264 suppliers
inquiry

487041-08-7
44 suppliers
inquiry

1056039-83-8
125 suppliers
$16.00/250mg

856866-72-3
264 suppliers
inquiry