
4,6-Dichloro-5-pyrimidinecarbaldehyde synthesis
- Product Name:4,6-Dichloro-5-pyrimidinecarbaldehyde
- CAS Number:5305-40-8
- Molecular formula:C5H2Cl2N2O
- Molecular Weight:176.99
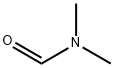
68-12-2
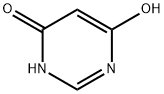
1193-24-4

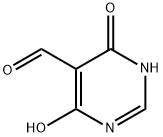
5305-40-8
General procedure for the synthesis of 4,6-dichloro-5-pyrimidinecarboxaldehyde from N,N-dimethylformamide (DMF) and 4,6-dihydroxypyrimidine: First, DMF (64 mL) was mixed with phosphorus oxychloride (POCl3) (200 mL) and stirred for 1 hr at 0 °C. Subsequently, 4,6-dihydroxypyrimidine (50.0 g, 446 mmol) was added to the mixture and stirring was continued for 0.5 h at room temperature. Next, the resulting non-homogeneous mixture was heated to reflux for 3 hours. After completion of the reaction, the volatiles were removed by distillation under reduced pressure and the residue was carefully poured into ice water. The aqueous phase was extracted six times with ether and the organic phases were combined. The organic phase was washed sequentially with aqueous sodium bicarbonate (NaHCO3) and pure water and dried over anhydrous sodium sulfate (Na2SO4). The dried organic phase was concentrated under reduced pressure and finally crystallized by ethyl acetate (EtOAc)-petroleum ether mixed solvent to afford the target product 4,6-dichloro-5-pyrimidinecarboxaldehyde (43.5 g, 55% yield). The product was analyzed by LC-MS (ESI), m/z 177 [M+H]+.

1193-24-4
568 suppliers
$6.00/25g

5305-40-8
329 suppliers
$10.00/1g
Yield:5305-40-8 95%
Reaction Conditions:
Stage #1:N,N-dimethyl-formamide with trichlorophosphate at 0; for 1 h;
Stage #2:4,6-pyrimidinediol at 20; for 0.5 h;
Steps:
1.a
EXAMPLE 1; 4-[6-Amino-5-(methoxyimino-methyl)-pyrimidin-4-yl]-piperazine-l-carboxylic acid (4-isopropoxy-phenyl)-amidea. 4,6-Dichloro-pyrimidine-5-carbaldehyde; A mixture of DMF (3.2 mL) and POCl3 (10 mL) at 0 0C was stirred for 1 h, treated with 4,6-dihydroxypyrimidine (2.5 g, 22.3 mmol), and stirred for 0.5 h at ambient EPO
References:
JANSSEN PHARMACEUTICA N.V. WO2006/135719, 2006, A1 Location in patent:Page/Page column 46-47
14256-99-6
75 suppliers
$45.00/10mg

5305-40-8
329 suppliers
$10.00/1g