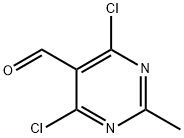
4,6-DICHLORO-2-METHYLPYRIMIDINE-5-CARBALDEHYDE synthesis
- Product Name:4,6-DICHLORO-2-METHYLPYRIMIDINE-5-CARBALDEHYDE
- CAS Number:14160-91-9
- Molecular formula:C6H4Cl2N2O
- Molecular Weight:191.01

15263-76-0

14160-91-9
The general procedure for the synthesis of 2-methyl-4,6-dichloropyrimidine-5-carbaldehyde from 2N-(4-((3-propylamino)amino)phenyl)-4N-(5-cyclopropane-1H-pyrazolyl)pyrimidine-2,4-diamine hydrochloride is as follows: Intermediate 45: Synthesis of 4,6-dichloro-2-methylpyrimidine-5-carbaldehyde 1. water (0.199 mL, 11.03 mmol) was slowly added to phosphorus trichloride (7.28 mL, 78.11 mmol) under ice bath conditions. 2. N,N-dimethylaniline (0.291 mL, 2.30 mmol) was then added. 3. N-((4,6-dihydroxy-2-methylpyrimidin-5-yl)methylene)-N-methylmethylammonium chloride (Intermediate 46; 1 g, 4.59 mmol) was added to the above solution in batches at room temperature. 4. The resulting suspension was stirred and reacted at 120 °C for 20 hours. 5. Upon completion of the reaction, the reaction mixture was slowly poured into an ice/chloroform mixture. 6. The chloroform layer was washed sequentially with saturated NaHCO3 solution (200 mL) and saturated brine (100 mL). 7. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the crude product. 8. The crude product was purified by fast silica gel column chromatography with an elution gradient of 5% to 10% EtOAc in isohexane solution. 9. The pure grades were collected and concentrated to dryness under reduced pressure to give 2-methyl-4,6-dichloropyrimidine-5-carbaldehyde (0.160 g, yield 18.23%) as a white solid. Product characterization: 1H NMR (400 MHz, CDCl3) δ 2.77 (3H, s), 10.44 (1H, s).

1194-22-5
135 suppliers
$6.00/5g

14160-91-9
122 suppliers
$19.00/100mg
Yield:14160-91-9 49.5%
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-formamide at 0 - 120; for 3 h;
Steps:
1.A Step A. 4,6-dichloro-2-methylpyrimidine-5-carbaldehyde.
A stirred mixture of DMF (6.2 mL, 39.26 mmol) was added POCI3 (100 g, 652 mmol) at 0 °C. After stirred at rt for 10 min. 2-methylpyrimidine-4,6-diol (10 g, 79.3 mmol) was added at 0 °C. After stirred at 120 °C for 3h, the cooled mixture was concentrated. The crude product was poured into cooled NaHCO3 (100 mL, aq) and extracted with EtOAc twice. The combined organic phase was washed with NaHCO3 (100 mL, aq), brine, dried over Na2SO4, filtered and concentrated. The crude product was purified by chromatography (silica gel, 0-20 %, EtOAc in PE) to give 4,6-dichloro-2-methylpyrimidine-5-carbaldehyde (7.5 g, 39 mmol, 49.5 %) as a white solid. LC-MS (ESI) m/z 190 (M+H)+.
References:
WO2022/187236,2022,A1 Location in patent:Paragraph 0209

15263-76-0
1 suppliers
inquiry

14160-91-9
122 suppliers
$19.00/100mg
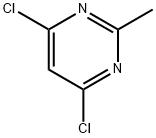
1780-26-3
476 suppliers
$10.00/10g
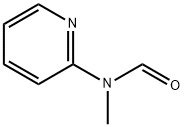
67242-59-5
77 suppliers
$22.39/250MG

14160-91-9
122 suppliers
$19.00/100mg