
2,4-DICHLORO-5-NITROPYRIDINE synthesis
- Product Name:2,4-DICHLORO-5-NITROPYRIDINE
- CAS Number:4487-56-3
- Molecular formula:C5H2Cl2N2O2
- Molecular Weight:192.99
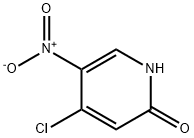
850663-54-6

4487-56-3
Step 3. Synthesis of 2,4-dichloro-5-nitropyridine: The product of step 2, 2-hydroxy-4-chloro-5-nitropyridine (40.0 g, 229 mmol), was suspended in toluene (300 mL), and phosphorus oxychloride (POCl3, 65 mL, 697 mmol) was added dropwise over a period of 10 min. Subsequently, the reaction mixture was heated to reflux and maintained for 6 hours. Upon completion of the reaction, the mixture was cooled to 60 °C and stirred continuously at this temperature overnight. The non-homogeneous reaction mixture was cooled to room temperature and subsequently concentrated under reduced pressure to remove the solvent. The residue was carefully adjusted to an alkaline pH with saturated aqueous potassium carbonate (K2CO3). extraction was carried out with ethyl acetate (EtOAc), the organic phases were combined and washed sequentially with water and saturated brine. The organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered and the filtrate was concentrated to give the crude product as an oil. Purification by silica gel column chromatography (eluent: hexane solution of 50% ethyl acetate) afforded the title compound 2,4-dichloro-5-nitropyridine (32.5 g, 74% yield) as an orange oil, which solidified to a solid on standing. Mass spectrometry (electrospray positive ion mode, ES+) showed the molecular ion peak m/e 194 [M+H]+.
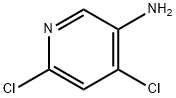
7321-93-9
162 suppliers
$20.00/100mg

4487-56-3
291 suppliers
$24.00/1g
Yield:4487-56-3 77%
Reaction Conditions:
with sulfuric acid;dihydrogen peroxide in water at 0 - 25; for 18 h;
Steps:
1 Synthesis of 2,4-dichloro-5-nitro-pyridine
Fuming H2SO4 (13 mL) was added dropwise to the stirred vessel. In a separate flask, concentrated H2SO4 was added to 4,6-dichloro-pyridin-3-ylamine (4.54 g, 27.9 mmol) and stirred until complete dissolution occurred. The amine solution was then added to the H2O2/fuming H2SO4 solution, dropwise. The reaction was allowed to warm to 25° C. over 18 h. The yellow solution was poured over ice and neutralized by the slow addition of solid NaHCO3. The resultant aqueous solution was extracted three times with EtOAc and the combined organic layers were dried (Na2SO4), decanted and concentrated to afford 2,4-dichloro-5-nitro-pyridine as a yellow solid (4.13 g, 77%).
References:
Boehringer Ingelheim International GmbH US2006/217417, 2006, A1 Location in patent:Page/Page column 13

850663-54-6
135 suppliers
$31.00/250mg

4487-56-3
291 suppliers
$24.00/1g

24484-96-6
160 suppliers
$11.00/250mg

4487-56-3
291 suppliers
$24.00/1g

13091-23-1
343 suppliers
$14.14/1gm:

4487-56-3
291 suppliers
$24.00/1g
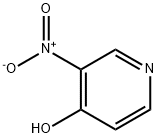
5435-54-1
367 suppliers
$19.00/25g

4487-56-3
291 suppliers
$24.00/1g