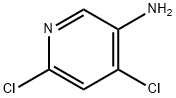
4,6-DICHLORO-PYRIDIN-3-YLAMINE synthesis
- Product Name:4,6-DICHLORO-PYRIDIN-3-YLAMINE
- CAS Number:7321-93-9
- Molecular formula:C5H4Cl2N2
- Molecular Weight:163

70593-57-6

7321-93-9
The general procedure for the synthesis of 2,4-dichloro-5-aminopyridine from 4,6-dichloropyridin-3-amide was as follows: sodium hydroxide (6.60 g, 165 mmol) was dissolved in water (31 mL) and cooled in an ice bath. Subsequently, bromine (2.08 mL, 40.6 mmol) was added dropwise and the resulting yellow solution was stirred for 15 minutes. A 1,4-dioxane (21 mL) solution of 4,6-dichloronicotinamide (7.27 g, 38.1 mmol) was slowly added dropwise to the above bromine solution over 30 minutes. The reaction mixture was allowed to gradually warm up to 25°C over 18 hours. Upon completion of the reaction, the volatiles were removed by distillation under reduced pressure and the remaining solution was diluted with brine and poured into ethyl acetate. The aqueous phase was separated and extracted twice with ethyl acetate. The organic layers were combined, dried with sodium sulfate, decanted and concentrated to give an orange colored oil. The oily substance was purified by passing through a 100 g silica gel fast column using 25% ethyl acetate-hexane as eluent to give 4,6-dichloropyridin-3-ylamine as a brown solid (4.54 g, 73% yield). Finally, 30% aqueous hydrogen peroxide solution (29 mL) was cooled in an ice bath.

70593-57-6
90 suppliers
$10.00/250mg

7321-93-9
162 suppliers
$20.00/100mg
Yield:7321-93-9 73%
Reaction Conditions:
with sodium hydroxide;water;bromine in 1,4-dioxane at 0 - 25; for 18 h;
Steps:
1 Synthesis of 2,4-dichloro-5-nitro-pyridine
NaOH (6.60 g, 165 mmol) was dissolved in H2O (31 mL) and cooled in an ice bath. Bromine (2.08 mL, 40.6 mmol) was added dropwise and the yellow solution was stirred for 15 min. 4,6-Dichloro-nicotinamide (7.27 g, 38.1 mmol) in 1,4-dioxane (21 mL) was added dropwise to the bromine solution over 30 min. The reaction was allowed to warm slowly to 25° C. over 18 h. The volatiles were removed in vacuo and the resultant solution was diluted with brine and poured into EtOAc. The aqueous phase was separated and extracted twice with EtOAc. The organic layers were combined, dried (Na2SO4), decanted and concentrated to afford an orange oil. The resultant oil was purified on a 100 g SiO2 flash chromatography cartridge with 25 % EtOAc-hexanes to afford 4,6-dichloro-pyridin-3-ylamine as a tan solid (4.54 g, 73%). 30% Aqueous H2O2 solution (29 mL) was cooled in an ice bath.
References:
US2006/217417,2006,A1 Location in patent:Page/Page column 13

849937-96-8
159 suppliers
$35.00/1g

7321-93-9
162 suppliers
$20.00/100mg

4487-56-3
291 suppliers
$24.00/1g

7321-93-9
162 suppliers
$20.00/100mg

73027-79-9
272 suppliers
$13.00/1g

7321-93-9
162 suppliers
$20.00/100mg

65973-52-6
248 suppliers
$6.00/1g

7321-93-9
162 suppliers
$20.00/100mg