
2,6-Dichloro-3-nitropyridine synthesis
- Product Name:2,6-Dichloro-3-nitropyridine
- CAS Number:16013-85-7
- Molecular formula:C5H2Cl2N2O2
- Molecular Weight:192.99
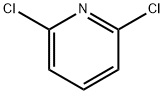
2402-78-0

16013-85-7
General procedure for the synthesis of 2,6-dichloro-3-nitropyridine from 2,6-dichloropyridine: 80 mL of concentrated sulfuric acid was added to a 150 mL three-necked flask with stirring at room temperature, and 7.4 g (0.05 mol) of 2,6-dichloropyridine (IV) was slowly added, followed by the slow addition of 10.1 g (0.1 mol) of potassium nitrate. After the addition was completed, stirring was continued for 30 minutes, then the temperature was slowly increased to 120°C and the reaction was maintained at this temperature for 10 hours. Upon completion of the reaction, the reaction mixture was cooled to room temperature and poured into crushed ice with stirring. The precipitated white solid precipitate was washed to neutrality with ice water, immediately subjected to diafiltration, and dried to give 7.75 g of white solid 2,6-dichloro-3-nitropyridine (V) in 80% yield and melting point of 61-63 °C.
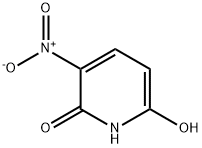
16013-84-6
26 suppliers
inquiry

16013-85-7
503 suppliers
$9.00/5g
Yield:16013-85-7 92.9%
Reaction Conditions:
with bis(trichloromethyl) carbonate in N,N-dimethyl-formamide at 80 - 85; for 6 h;Solvent;Temperature;Reagent/catalyst;
Steps:
5-7 Example 7: Preparation of 2,6-dichloro-3-nitropyridine
To a 250 ml four-necked flask equipped with a thermometer, mechanical stirring, and reflux condenser, 70 g of N,N-dimethylformamide was added.31.2 g (0.2 mol) of 2,6-dihydroxy-3-nitropyridine,38.6 g (0.13 mol) of triphosgene, stirring reaction at 80-85 ° C for 6 hours,Cool to 20-25 ° C,Slowly pour the residue into 200 grams of ice water.Extract three times with chloroform, 50 grams each time.The organic phases were combined and washed with 30 g of saturated brine.Then dried with 5 g of anhydrous sodium sulfate.The solvent was removed by rotary evaporation to give 35.5 g, m.The yield is 92.9%.The liquid phase purity was 99.3%
References:
CN110218177,2019,A Location in patent:Paragraph 0040-0045

2402-78-0
524 suppliers
$6.00/25g

16013-85-7
503 suppliers
$9.00/5g
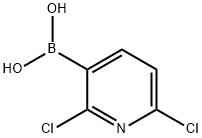
148493-34-9
220 suppliers
$6.00/250mg

16013-85-7
503 suppliers
$9.00/5g
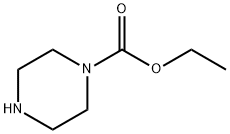
120-43-4
284 suppliers
$6.00/5g

16013-85-7
503 suppliers
$9.00/5g

92741-37-2
0 suppliers
inquiry

75167-21-4
0 suppliers
inquiry

92741-38-3
0 suppliers
inquiry