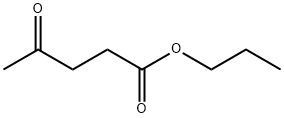
Propyl Levulinate synthesis
- Product Name:Propyl Levulinate
- CAS Number:645-67-0
- Molecular formula:C8H14O3
- Molecular Weight:158.2
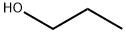
71-23-8
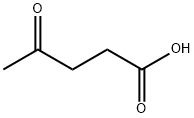
123-76-2

645-67-0
General procedure for the synthesis of propyl 4-oxopentanoate from n-propanol and acetylpropionic acid: the esterification of acetylpropionic acid was carried out in a 50 mL round-bottomed flask fitted with a reflux condenser tube. In a typical catalyzed reaction, catalyst (40 mg) was added to a mixture of acetylpropionic acid and n-propanol, where the molar ratio of acetylpropionic acid to n-propanol was 1:8 (with n-propanol acting as both the reactant and the solvent), and the mixture was subsequently magnetically stirred for 2 hours at 333 K. The reaction was carried out in a 50 mL round bottom flask equipped with a reflux condenser tube. After the reaction reached the predetermined time, a portion of the reaction mixture was taken and separated by filtration and the filtrate was analyzed by gas chromatography (GC) equipped with a flame ionization detector and a capillary column. All the compounds were characterized by their spectral data (1H NMR) and compared with the data reported in literature.
Yield:645-67-0 97.76%
Reaction Conditions:
with SO42-/MxOy in water at 20 - 80; for 5 h;Temperature;Solvent;Reagent/catalyst;
Steps:
5; 6 Example 6 Preparation of propyl levulinate:
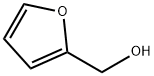
After mixing 50 grams of furfuryl alcohol, 375 grams of propanol, 10 grams of water, and 20 grams of SO42- / MxOy super strong acid, the reaction is carried out at a temperature of 80 ° C, and the stirring speed is controlled to 600 rpm, the condensation reflux temperature is 20 ° C, the reaction After 5 hours, after the temperature was lowered to room temperature, the catalyst was removed by filtration, and the propanol in the reaction mixture was distilled off under reduced pressure at 45 ° C to obtain light yellow propyl levulinate, which was analyzed by high performance liquid chromatography, the conversion rate of furfuryl alcohol and acetyl The yields of propyl propionate were 100% and 97.76%, respectively, and the yield was 97.76%.
References:
CN110963914,2020,A Location in patent:Paragraph 0054-0057
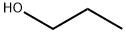
71-23-8
793 suppliers
$12.00/100g

539-88-8
366 suppliers
$5.00/5g

645-67-0
65 suppliers
$6.00/1g

108-29-2
376 suppliers
$10.00/5G
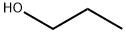
71-23-8
793 suppliers
$12.00/100g

123-76-2
495 suppliers
$5.00/25g
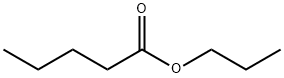
141-06-0
32 suppliers
$450.55/5MG

645-67-0
65 suppliers
$6.00/1g

108-29-2
376 suppliers
$10.00/5G