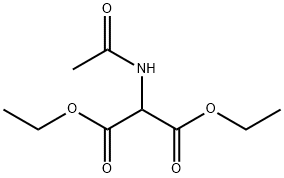
Diethyl acetamidomalonate synthesis
- Product Name:Diethyl acetamidomalonate
- CAS Number:1068-90-2
- Molecular formula:C9H15NO5
- Molecular Weight:217.22
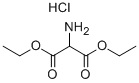
13433-00-6

75-36-5

1068-90-2
Diethyl 2-aminomalonate hydrochloride (1.69 g, 8.0 mmol) and triethylamine (3.4 mL, 24 mmol, 3.0 eq.) were dissolved in dichloromethane (120 mL) at 0 °C. Acetyl chloride (0.57 mL, 8.0 mmol, 1.0 eq.) was added slowly and dropwise with stirring and the reaction mixture was gradually warmed to room temperature and stirred overnight. After completion of the reaction, the mixture was diluted with dichloromethane (100 mL) and washed sequentially with 1 M hydrochloric acid (3 x 60 mL). The aqueous phase was then back-extracted with dichloromethane (2 x 60 mL), the organic layers were combined and dried over anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to give a pure white solid product. Yield: 1.68 g (96% yield). The product was characterized as follows: melting point 96 °C (literature value 97 °C); 1H NMR (300 MHz, CDCl3) δ [ppm]: 1.30 (t, J=7.1Hz, 6H, 7-CH3,9-CH3), 2.08 (s, 3H, 5-CH3), 4.27 (m, 4H, 6-CH2,8-CH2), 5.18 (d, J=7.1Hz, 1H), 5.18 (d, J=7.1Hz, 1H). 7.1 Hz, 1H, 2-CH), 6.67 (d, J=5.7 Hz, 1H, 2-NH); 13C NMR (75 MHz, CDCl3) δ [ppm]: 14.0 (q, C-7, C-9), 22.8 (q, C-5), 56.5 (d, C-2), 62.6 (t, C-6, C-8), 166.5 (s, C -1, C-3), 169.9 (s, C-4); high-resolution mass spectrometry (ESI+): 240.0842 calculated value for C9H15N5O+Na+, 240.0824 measured value.
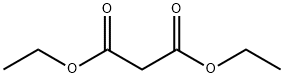
105-53-3
795 suppliers
$5.00/25g
123-91-1
714 suppliers
$16.00/25g

1068-90-2
568 suppliers
$5.00/25g
Yield:1068-90-2 86%
Reaction Conditions:
with acetic acid;sodium nitrite in water
Steps:
5 Example 5
Example 5 A mixture of 1,4-dioxane, acetic acid and water, obtained from a previous reaction mixture by taking a low-boiling cut, was metered into a stirred mixture of 320.4 g (2.0 mol) of diethyl malonate and 160 g (2.3 mol) of sodium nitrite (technical grade), maintained at 35° C. 12.0 g of water were then metered in and 166 g (207. mol) of acetic acid (96%) were added dropwise over 2 hours. The mixture was allowed to continue reacting for 2 hours at 40° C. and was worked up as described in Example 4. There remained 425 g of light yellow oil, which, after catalytic hydrogenation and recrystallization of the crude product, gave diethyl acetaminomalonate at a yield of 86% of theory, based on the diethyl malonate employed, and with a purity of >99.8 FID area percent.
References:
Huels Aktiengesellschaft US5861533, 1999, A

13433-00-6
487 suppliers
$9.00/1g

75-36-5
592 suppliers
$17.92/100G

1068-90-2
568 suppliers
$5.00/25g

13433-00-6
487 suppliers
$9.00/1g
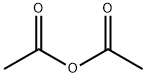
108-24-7
0 suppliers
$14.00/250ML

1068-90-2
568 suppliers
$5.00/25g
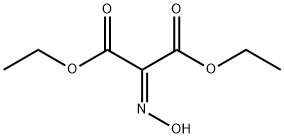
6829-41-0
37 suppliers
$199.00/500mg

1068-90-2
568 suppliers
$5.00/25g
