
Pimobendan synthesis
- Product Name:Pimobendan
- CAS Number:74150-27-9
- Molecular formula:C19H18N4O2
- Molecular Weight:334.37
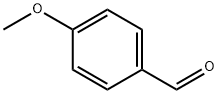
123-11-5

136609-85-3

118428-37-8
A mixture of 6-(3,4-diaminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one (II, 1.0 eq.) and sodium bisulfite (1.1 eq.) in DMF (3.5 L/kg; V:m(n)) was prepared. The mixture was heated to 110-115 °C and 4-methoxybenzaldehyde (1.0 eq.) was slowly added over 30 minutes with stirring. After addition, stirring was continued for 60 minutes. Subsequently, the suspension was cooled to 60-70 °C, filtered and the filter cake was washed with DMF (1.24 L/kg; V:m(n)). The clarified filtrate was adjusted to pH 8-9 with ammonia and the temperature was maintained at 45-55°C. Next, the mixture was cooled to 25-30 °C and water (6.87 L/kg; V:m(n)) was added slowly with stirring. Stirring was continued for 1-2 hours at room temperature and the precipitated product was separated by filtration. The solid was washed sequentially with water (4 x 1.24 L/kg; V:m(n)) and acetone (4 x 2.47 L/kg; V:m(n)) and dried under reduced pressure at 40-60 °C to afford 6-(2-(4-methoxyphenyl)-1H-benzo[d]imidazol-5-yl)-5-methyl-4,5-dihydropyridazin-3(2H)-one monohydrate in 93.8% yield . Product analysis: residual water content 4.97% (KF method), HPLC purity 99.04%, melting point 162-164°C. The nuclear magnetic resonance hydrogen (1H-NMR, DMSO-d6, 300 MHz) and carbon (13C-NMR, DMSO-d6, 75 MHz) spectral and infrared (IR, KBr) spectral data were consistent with the structure of the target compound.

123-11-5
875 suppliers
$10.00/10g

74150-02-0
83 suppliers
inquiry

74150-27-9
324 suppliers
$5.00/10mg
Yield:74150-27-9 93.8%
Reaction Conditions:
with sodium metabisulfite in N,N-dimethyl-formamide at 110 - 115; for 1.5 h;Product distribution / selectivity;
Steps:
4.4.A.1
A reactor was charged with 6-(3,4-diaminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one (II, 1 .0 eq.), prepared according to example 3, and sodium metabisulfite (1.1 eq.) in DMF (3.5 L/kg; V:m(n)). The mixture was heated to 1 10 - 1 15 °C and 4-Methoxybenzaldehyde (1 .0 eq.) was added over 30 min with stirring. After the addition was complete stirring was continued for a further 60 min. The suspension was cooled to 60 - 70 °C, filtered and the filter cake was washed with DMF (1 .24 L/kg; V:m(n)). The clear filtrate was treated with aq. ammonia at 45 - 55 °C to adjust the pH to a value of 8 - 9. The mixture was then cooled to 25 - 30 °C and water (6.87 L/kg; V:m(n)) was added slowly with stirring. Stirring was continued for a further 1 - 2 h at room temperature and the precipitated product was separated by filtration. The solid was washed with water (4 x 1.24 L/kg; V:m(n)) and acetone (4 x 2.47 L/kg; V:m(n)) and dried under reduced pressure at 40 - 60 °C to obtain 93.8 % of crystalline 6-(2-(4-methoxyphenyl)- 1 H-benzo[d]imidazol-5-yl)-5-methyl-4,5-dihydro-pyrida-zin-3(2H)-one monohydrate. Residual solvents: H20, 4.97 % (KF). HPLC: 99.04 %. Mp: 162-164 °C. 1H-NMR (DMSO, 300 MHz): δ = 12.82 (s, br. 1 H, NH), 10.89 (s, 1 H, NH), 8.14 - 8.1 1 (d, 2 H, 2 x CH, arom), 7.93 (s, 1 H, arom), 7.71 -7.68 (1 H, d, arom.), 7.59 - 7.56 (1 d, H, arom.), 7.13 - 7.10 (d, 2 H, arom.), 3.83 (s, 3 H, CH30), 3.53 - 3.44 (m, 1 H, CH), 3.56 (s, br), 2.87-2.68 (dd, 1 H, CH2), 2.28 - 2.22 (dd, 1 H, CH2), 1.13 - 1 .1 1 (d, 3 H, CH3). C-NMR (DMSO, 75 MHz): δ = 166.23, 160.76, 153.31 , 152.58, 128.78, 128.09, 122.37, 1 19.98, 1 14.37, 55.30, 33.65, 27.45, 16.02. IR (KBr, cm"1): 3273, 1620, 1493, 1459, 1422, 1393, 1380, 1342, 1297, 1252, 1 179, 1079, 1034, 960, 924, 893, 833, 813, 773, 744, 687, 639, 608, 519.
References:
WO2011/124638,2011,A1 Location in patent:Page/Page column 22-23
85633-96-1
26 suppliers
inquiry

74150-27-9
324 suppliers
$5.00/10mg

85633-97-2
6 suppliers
inquiry

74150-27-9
324 suppliers
$5.00/10mg
104-88-1
640 suppliers
$5.00/25g

74150-27-9
324 suppliers
$5.00/10mg

151870-45-0
0 suppliers
inquiry

74150-27-9
324 suppliers
$5.00/10mg