
3(2H)-Pyridazinone synthesis
- Product Name:3(2H)-Pyridazinone
- CAS Number:504-30-3
- Molecular formula:C4H4N2O
- Molecular Weight:96.09

110-89-4

60-29-7

106-89-8
![3(2H)-Pyridazinone, 4,5-dihydro-6-[4-hydroxy-3-(2-propen-1-yl)phenyl]-](/CAS/20210305/GIF/56872-17-4.gif)
56872-17-4

504-30-3
![3(2H)-Pyridazinone, 4,5-dihydro-6-[4-(2-oxiranylmethoxy)-3-(2-propen-1-yl)phenyl]-](/CAS/20210305/GIF/56872-18-5.gif)
56872-18-5
III. A finely ground mixture of 6-(3-allyl-4-hydroxyphenyl)-4,5-dihydro-3(2H)-pyridazinone (50 g, 0.22 mol), epichlorohydrin (200 g, 2.2 mol) and piperidine (2 g) was heated on a steam bath for 90 minutes. Upon completion of the reaction, the volatile components were removed by evaporation under reduced pressure to give a viscous oily substance. The oily substance was dissolved in dichloromethane and subsequently washed with dilute sodium hydroxide solution (500 mL) with agitation for 10 minutes. The organic phase was separated, washed with water, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain the oily substance, which gradually solidified during standing. Addition of ethanol ether to the solid afforded 6-[3-allyl-4-(2,3-epoxypropoxy)phenyl]-4,5-dihydro-3(2H)-pyridazinone (30 g, 47% yield, melting point 92.5°-95°C). The pure pyridazinone (melting point 93.5°-95°C) was further obtained by recrystallization from aqueous ethanol. The results of the elemental analyses were as follows: measured values (C, 67.41; H, 6.47; N, 9.80; M+, 286), which are in agreement with the theoretical values (C16H18N2O3: C, 67.11; H, 6.33; N, 9.79; M, 286).

57697-64-0
1 suppliers
inquiry

504-30-3
243 suppliers
$6.00/1g
Yield:504-30-3 88.9%
Reaction Conditions:
Stage #1: mucochloric acidwith tert-butylhydrazine in water;toluene at 23; for 2.8 h;
Stage #2: with tetrabutylammomium bromide;titanium(IV) oxide in water;toluene at 20; for 4.6 h;Temperature;
Steps:
1-10 Example 9
Add 50g furochloric acid and 9g toluene into a four-necked flask. Under stirring, 23 °C slowly drop a 20% aqueous solution of tert-butylhydrazine (28.4g of tert-butylhydrazine). After the addition, keep it warm for 2.8 h. Add 3.5g of C-TiO2 solid acid and 2.5g of tetrabutylammonium bromide, control the reaction temperature at 20 °C and react for 4.6 h. After the reaction is over, the solid acid catalyst is filtered out and reused. The filtrate was left standing to separate the organic phase and the inorganic phase, and the organic layer was decompressed to recover the solvent toluene to obtain the product pyridazinone. The calculated yield was 88.9%, and the product purity was 96.8% analyzed.
References:
CN112552241,2021,A Location in patent:Paragraph 0022-0031
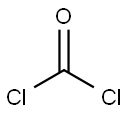
75-44-5
0 suppliers
inquiry

145743-48-2
1 suppliers
inquiry

504-30-3
243 suppliers
$6.00/1g

145743-63-1
11 suppliers
inquiry

106-95-6
435 suppliers
$10.00/5g

39499-64-4
0 suppliers
inquiry

504-30-3
243 suppliers
$6.00/1g
![3(2H)-Pyridazinone, 4,5-dihydro-6-[3-(2-propen-1-yloxy)phenyl]-](/CAS/20210305/GIF/56872-13-0.gif)
56872-13-0
0 suppliers
inquiry
![8aH-1,2,4-Oxadiazolo[4,5-b]pyridazine, 3-phenyl-](/CAS/20210305/GIF/178047-61-5.gif)
178047-61-5
0 suppliers
inquiry

504-30-3
243 suppliers
$6.00/1g

