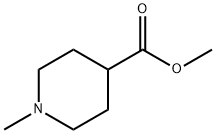
N-METHYL-4-PIPERIDINECARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:N-METHYL-4-PIPERIDINECARBOXYLIC ACID METHYL ESTER
- CAS Number:1690-75-1
- Molecular formula:C8H15NO2
- Molecular Weight:157.21

67-56-1
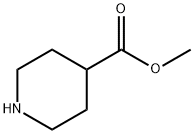
2971-79-1

1690-75-1
To a stirred solution of methyl 4-piperidinecarboxylate (7.00 mmol) in methanol (25 mL) was sequentially added formic acid (5.52 g, 12.00 mmol) and 40% aqueous formaldehyde (2.67 g, 35.00 mmol). The reaction mixture was heated to reflux. after 3 h, the reaction mixture was cooled and concentrated under reduced pressure. The residue was dissolved in water, alkalized with sodium bicarbonate solution and extracted with dichloromethane (3 x 30 mL). The organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give methyl N-methyl-4-piperidinecarboxylate in brown liquid form (yield: 910 mg). NMR hydrogen spectrum (270 MHz, CDCl3, TMS as internal standard) δ: 1.69-2.03 (6H, m), 2.23-2.32 (4H, m), 2.78-2.83 (2H, m), 3.68 (3H, s). Mass spectrum (APCI) m/z: 158 ([M+H]+, 100%), 126 (C7H12ON, 33%).

67-56-1
782 suppliers
$7.29/5ml-f

2971-79-1
272 suppliers
$10.00/10g

1690-75-1
150 suppliers
$8.00/5g
Yield:1690-75-1 91%
Reaction Conditions:
with formaldehyd;formic acid in water for 3 h;Heating / reflux;
Steps:
To a stirred solution of methyl isonipecotate (L. OOG, 7.00 mmol) in methanol (25 ml) was added formic acid (5.52g, 12.00 mmol) and a solution of formaldehyde (2.67g, 35.00 mmol, 40% aqueous) and the reaction heated at reflux. After 3 hours the reaction was cooled and concentrated under reduced pressure. The aqueous solution was basified with NAHC03 and extracted with DCM (3 x 30 ml), dried (MGSO4) and concentrated under reduced pressure to give a brown liquid (L. OOG, 910-.) ; b (270 MHz; CDC13 ; Me4Si), 1.69-2. 03 (6 H, m), 2.23-2. 32 (4H, m), 2.78- 2.83 (2H, m), 3.68 (3 H, s); M/Z (APCI) 158 (100% [M+H] +), 126 (33, C7H120N).
References:
BIOFOCUS PLC WO2004/58259, 2004, A1 Location in patent:Page/Page column 26-27

67-56-1
782 suppliers
$7.29/5ml-f
71985-80-3
159 suppliers
$6.00/1g

1690-75-1
150 suppliers
$8.00/5g

71985-80-3
159 suppliers
$6.00/1g

1690-75-1
150 suppliers
$8.00/5g

2971-79-1
272 suppliers
$10.00/10g

1690-75-1
150 suppliers
$8.00/5g
7630-02-6
7 suppliers
inquiry

1690-75-1
150 suppliers
$8.00/5g