
1-Methyl-4-piperidinemethanol synthesis
- Product Name:1-Methyl-4-piperidinemethanol
- CAS Number:20691-89-8
- Molecular formula:C7H15NO
- Molecular Weight:129.2

24252-37-7

20691-89-8
The general procedure for the synthesis of 1-methyl-4-piperidinemethanol from ethyl N-methyl-4-piperidinecarboxylate is as follows: Synthesis of Example 23 (1-methylpiperidin-4-yl)methanol (22): a solution of ethyl N-methyl-4-piperidinecarboxylate (21, 10.1 g, 59.0 mmol) in ethyl ether (Et2O, 40 mL) was slowly added dropwise to an ethyl ether suspension of lithium aluminum hydride (LiAlH4, 2.46 g, 64.9 mmol). The reaction system was cooled at 0 °C, followed by the addition of ether (200 mL). The reaction mixture was gradually warmed to room temperature and stirred continuously at this temperature for 4 hours. Upon completion of the reaction, the reaction was quenched by the slow addition of water (10 mL) and stirring was continued for 30 minutes. The resulting white precipitate was separated by filtration and washed well with ether (250 mL). After concentration under reduced pressure to remove the solvent, the oily product obtained was purified by distillation under reduced pressure (boiling point 108 °C, 14 mbar) to give the target compound 22 (6.40 g, 84% yield) as a colorless oil. -Thin layer chromatography (TLC) analysis: Rf = 0.53 (Expanding agent: dichloromethane/methanol = 5:1 with 5% triethylamine, phosphomolybdic acid color development: dark blue spots). -Nuclear magnetic resonance hydrogen spectrum (1H NMR, 300MHz, CDCl3): δ= 1.28 (dq, J = 12.2, 3.7Hz, 2H, 3-Hax, 5-Hax), 1.38-1.55 (m, 1H, 4-H), 1.68-1.79 (m, 2H, 3-Heq, 5-Heq), 1.93 (dt, J = 11.8, 2.3Hz, 2H, 2H, 4-H). 2.3 Hz, 2H, 2-Hax, 6-Hax), 2.26 (s, 3H, NMe), 2.82-2.92 (m, 2H, 2-Heq, 6-Heq), 3.12 (sbr, 1H, OH), 3.46 (d, J = 6.4 Hz, 2H, CH2OH) ppm. -Nuclear magnetic resonance carbon spectrum (13C NMR, 75.5 MHz, CDCl3): δ= 28.8 (C-3, C-5), 37.9 (C-4), 46.3 (NMe), 55.5 (C-2, C-6), 67.4 (CH2OH) ppm. -Mass spectrum (MS, 70eV, EI): m/z (%) = 129 (55) [M]+, 128 (100) [M-H]+, 112 (10) [M-OH]+, 98 (21) [M-CH2OH]+. -Molecular formula: C7H15NO (molecular weight: 129.20).

123855-51-6
415 suppliers
$6.00/5g

20691-89-8
231 suppliers
$6.00/1g
Yield:20691-89-8 88%
Reaction Conditions:
Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 20;Inert atmosphere;
Stage #2: with water;sodium hydroxide in tetrahydrofuran at 0 - 20;
Steps:
1 (1-Methylpiperidin-4-yl)methyl 4-nitrophenyl carbonate
A solution of tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (1.94 g, 9.0 mmol) in THF (15.0 mL) was added drop-wise to a 1M solution of LiAlH4 in THF (13.5 mL, 13.5 mmol) under argon. The reaction mixture was stirred at room temperature for 17 hours and then cooled to 0° C. A mixture of THF and water (1:1 ratio, 1.5 mL) was added drop-wise. A gelatinous white solid formed. 4M aq NaOH solution (0.6 mL) was added drop-wise. Water (2 mL) was added and the resulting mixture stirred at room temperature for 2 hours. The white solid was removed by filtration. The filtrate was loaded onto an Isolute HM-N liquid-liquid extraction column and then eluted with EtOAc (200 mL). The resulting organic phase concentrated in vacuo yielding (1-methylpiperidin-4-yl)methanol as a yellow oil (1.02 g, 88%). Analytical LCMS: purity ~90% (System B, RT=1.88 min), ES+: 129.8 [MH]+. (
References:
US2009/281087,2009,A1 Location in patent:Page/Page column 9-10

24252-37-7
176 suppliers
$6.00/1g

20691-89-8
231 suppliers
$6.00/1g
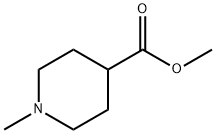
1690-75-1
150 suppliers
$8.00/5g

20691-89-8
231 suppliers
$6.00/1g
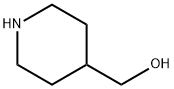
6457-49-4
367 suppliers
$18.00/25g

20691-89-8
231 suppliers
$6.00/1g
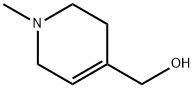
36166-75-3
30 suppliers
$130.00/50mg

20691-89-8
231 suppliers
$6.00/1g