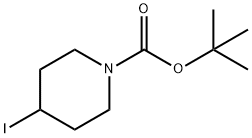
N-Boc-4-iodopiperidine synthesis
- Product Name:N-Boc-4-iodopiperidine
- CAS Number:301673-14-3
- Molecular formula:C10H18INO2
- Molecular Weight:311.16
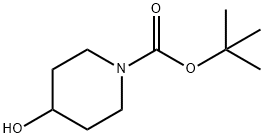
109384-19-2

301673-14-3
The general procedure for the synthesis of N-Boc-4-iodopiperidine from N-Boc-4-hydroxypiperidine was as follows: to a solution of N-Boc-4-hydroxypiperidine (10.0 g, 49.7 mmol) in dichloromethane (200 mL) was added triphenylphosphine (16.9 g, 64.6 mmol) and imidazole (5.07 g, 74.5 mmol) sequentially. After the reaction mixture was cooled to 0°C in an ice bath, iodine (15.1 g, 59.6 mmol) was added slowly in portions. After addition, the reaction mixture was stirred at room temperature for 18 hours. After completion of the reaction, the reaction solution was diluted with water and extracted with ether. The organic layers were combined, washed sequentially with water and saturated sodium chloride solution and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the residue was ground with hexane to remove excess triphenylphosphine and triphenylphosphine oxide. After filtration, the filtrate was concentrated under reduced pressure to afford the target product N-Boc-4-iodopiperidine as a colorless oil (14.4 g, 93% yield).

109384-19-2
435 suppliers
$5.00/1g

301673-14-3
210 suppliers
$10.00/1g
Yield:301673-14-3 93%
Reaction Conditions:
with 1H-imidazole;iodine;triphenylphosphine in dichloromethane at 0 - 20; for 18 h;
Steps:
24.A Part A. Preparation of iodo Intermediate
To a solution of N-Boc-4-hydroxypiperdine (10.0 g, 49.7 mmol) in dichloromethane (200 mL) was added triphenylphosphine (16.9 g, 64.6 mmol) and imidazole (5.07 g, 74.5 mmol). The resulting slurry was cooled to 0° C. in an ice bath. Iodine (15.1 g, 59.6 mmol) was added in small portions. The solution was then stirred for 18 hr at ambient temperature. Afterward, the solution was diluted with water and extracted with diethyl ether. The organic layer was washed with water and saturated aqueous NaCl, and then dried over sodium sulfate. Concentration in vacuo followed by trituration with hexane removed the excess triphenylphosphine and triphenylphosphine oxide. The filtrate was concentrated in vacuo to provide the iodo intermediate as a colorless oil (14.4 g, 93% yield).
References:
Barta, Thomas E.;Becker, Daniel P.;Bedell, Louis J.;Boehm, Terri L.;Brown, David L.;Carroll, Jeffery N.;Chen, Yiyuan;Fobian, Yvette M.;Freskos, John N.;Gasiecki, Alan F.;Grapperhaus, Margaret L.;Heintz, Robert M.;Hockerman, Susan L.;Kassab, Darren J.;Khanna, Ish K.;Kolodziej, Stephen A.;Massa, Mark A.;McDonald, Joseph J.;Mischke, Brent V.;Mischke, Deborah A.;Mullins, Patrick B.;Nagy, Mark A.;Norton, Monica B.;Rico, Joseph G.;Schmidt, Michelle A.;Stehle, Nathan W.;Talley, John J.;Vernier, William F.;Villamil, Clara I.;Wang, Lijuan J.;Wynn, Thomas A. US2005/9838, 2005, A1 Location in patent:Page 143
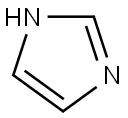
288-32-4
1042 suppliers
$5.00/25g

109384-19-2
435 suppliers
$5.00/1g

301673-14-3
210 suppliers
$10.00/1g
84358-13-4
378 suppliers
$5.00/5g

301673-14-3
210 suppliers
$10.00/1g
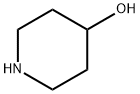
5382-16-1
0 suppliers
$9.00/5 g

301673-14-3
210 suppliers
$10.00/1g

24424-99-5
859 suppliers
$13.50/25G

301673-14-3
210 suppliers
$10.00/1g