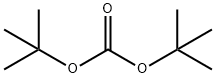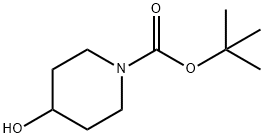
N-BOC-4-Hydroxypiperidine synthesis
- Product Name:N-BOC-4-Hydroxypiperidine
- CAS Number:109384-19-2
- Molecular formula:C10H19NO3
- Molecular Weight:201.26

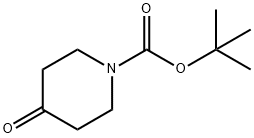
79099-07-3
0 suppliers
$5.00/5g

109384-19-2
445 suppliers
$5.00/1g
Yield:109384-19-2 100%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-onewith sodium tetrahydroborate in ethanol at 0 - 20; for 4 h;
Stage #2: with water;ammonium chloride in ethanol;
Steps:
38.1
Example 38 1st) Synthesis of tert-butyl 4-hydroxy-1-piperidinecarboxylate 1 1 g (5 mmol) of tert-butyl 4-oxo-1-piperidinecarboxylate (marketed by Aldrich) is dissolved in 5 ml of ethanol. This solution is cooled down to 0° C. using an ice bath and 200 mg (7.56 mmol) of sodium tetraborohydride is added by portions and the reaction mixture is stirred for 4 hours at ambient temperature. A saturated aqueous solution of ammonium chloride is added. The ethanol is evaporated off under reduced pressure (2 kPa) then the reaction mixture is taken up in ethyl acetate. The organic phase is separated from the aqueous phase. This extraction is repeated one more time and then the organic phases are combined and dried over magnesium sulphate, followed by concentrating under reduced pressure (2 kPa) and in this way 1.05 g (Yield=100%) of a colourless oil is recovered. TLC: Rf=0.5 (silicagel, eluent: CH2Cl2/MeOH 90:10 1H-NMR (CDCl3): δ 1.47 (s, 9H, tBu) and (m, 2H; -CH-CH2-N-CH2-CH-); 1.87 (m, 2H, -CH-CH2-N-CH2-CH-); 3.04 (m, 2H, -CH-N-CH-); 3.85 (m, 2H, -CH-N-CH-) and (m, H, -C-OH)
References:
US2006/52398,2006,A1 Location in patent:Page/Page column 62
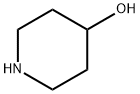
5382-16-1
0 suppliers
$9.00/5G

24424-99-5
864 suppliers
$13.50/25G

109384-19-2
445 suppliers
$5.00/1g

5382-16-1
0 suppliers
$9.00/5G

109384-19-2
445 suppliers
$5.00/1g

24424-99-5
864 suppliers
$13.50/25G

109384-19-2
445 suppliers
$5.00/1g