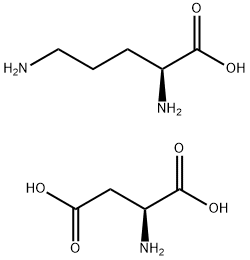
L-Ornithine L-aspartate salt synthesis
- Product Name:L-Ornithine L-aspartate salt
- CAS Number:3230-94-2
- Molecular formula:C5H12N2O2.C4H7NO4
- Molecular Weight:265.26

56-84-8
60259-81-6

3230-94-2
General procedure for the synthesis of L-ornithine L-aspartate salt from L-aspartate and L-ornithine acetate: 22.5 g of L-ornithine acetate (monohydrate) and 13.3 g of L-aspartate were added to 20 mL of purified water and mixed with stirring. Ammonium carbonate solution (prepared by dissolving 9.9 g of ammonium carbonate in 40 mL of pure water) was slowly added dropwise, with the rate of acceleration of the droplets being controlled in response to the accompanying slight exothermic and CO2 gas release. After observing that the suspension gradually became clarified, stirring was continued for 10 min to ensure that the pH of the reaction solution was maintained at 7.0-8.0. Subsequently, the reaction solution was heated to 70-80 °C, and 150 mL of methanol was slowly added to induce precipitation of solids. The crystallization process was continued for 2 hours, then slowly cooled to room temperature and stirring was continued for 2 hours. The solid was collected by filtration and washed once with a mixture of methanol and water (4:1 v/v, about 50 mL), then with pure methanol (50 mL x 2). After the above treatment, 21.7 g of wet solid product was obtained, which did not need to be dried and was directly used in the next step of refinement. Dissolve 21.7 g of aspartic ornithine wet crude product in 42 mL of pure water and stir until completely dissolved. It was heated to 60-70°C, and about 0.6 g of activated carbon was added and thermally decolorized for 30 minutes. After filtration, the filtrate was reheated to 70-80°C and about 84 mL of methanol was added slowly to induce precipitation of solids. After dropwise addition, stirring was continued for 2 hours, then slowly cooled to room temperature and stirred for another 2 hours. The solid was collected by filtration and washed sequentially with a mixture of methanol and water (4:1, 50 mL x 2, by volume) and pure methanol (50 mL x 2). Finally, 18.3 g of pure product was obtained by drying under reduced pressure with a specific rotation of +28.1° and a purity of 99.56%.
Yield:3230-94-2 115 g
Reaction Conditions:
with zinc(II) chloride; pH=8.3 at 35 - 40; for 1.5 h;Reagent/catalyst;Temperature;pH-value;
Steps:
1-3; 1; 2 Example 1
Dissolve 100 g of L-arginine in 800 ml of water, adjust the temperature to 35-40 ° C, add aspartic acid to adjust the pH to 8.3, add 0.2 g of zinc chloride, 20 g of immobilized arginase in sequence, and react 1.5 Hours, 0.1% arginine residue in the reaction solution was monitored by HPLC (see Table 1 for purity of lactam in the reaction solution), filtered, aspartic acid was added to the filtrate to adjust the pH to 6.9, and concentrated to about 400 mL under reduced pressure. 800 mL of methanol was added at 75 ° C and crystallized to obtain a crude aspartic acid ornithine.Add 300 mL of water to the crude aspartic acid ornithine, stir to dissolve, add 4 g of activated carbon, stir and filter, and add 900 mL of methanol at 70-75 ° C to crystallize, grow for 30 minutes, drop to normal temperature, filter, and Aspartic acid ornithine products
References:
Hebei Yipin Pharmaceutical Co., Ltd.;Zhang Ji;Jia Zongying;Zhao Cuiran;Bai Shanshan;Li Lu;Zhang Xiaocai;Zhang Yongran;Zhang Xiaoqian CN110627671, 2019, A Location in patent:Paragraph 0034-0053

56-84-8
930 suppliers
$5.00/25g

60259-81-6
130 suppliers
$10.00/5g

3230-94-2
441 suppliers
$13.00/25g
