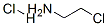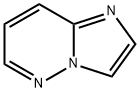
Imidazo[1,2-b]pyridazine synthesis
- Product Name:Imidazo[1,2-b]pyridazine
- CAS Number:766-55-2
- Molecular formula:C6H5N3
- Molecular Weight:119.12
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)
6775-78-6
![Imidazo[1,2-b]pyridazine](/CAS/GIF/766-55-2.gif)
766-55-2
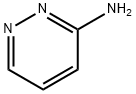
General procedure for the synthesis of imidazo[1,2-b]pyridazine from 6-chloroimidazo[1,2-b]pyridazine: to a stirred mixed solution of 6-chloroimidazo[1,2-b]pyridazine (800 mg, 5.21 mmol) in methanol (20 mL) and tetrahydrofuran (20 mL) was sequentially added triethylamine (0.8 mL, 5.74 mmol) and Pd/C ( 100 mg, 0.094 mmol). The reaction mixture was placed under hydrogen atmosphere and stirred for 16 hours. Upon completion of the reaction, the mixture was filtered through diatomaceous earth and the diatomaceous earth bed was washed with methanol. The filtrates were combined and concentrated, and the concentrate was subsequently suspended in water and extracted with ethyl acetate (3 x 20 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated to give imidazo[1,2-b]pyridazine (550 mg, 87% yield) as an off-white solid. The product was analyzed by LCMS [m/z 120 (M + H)]; 1H NMR (300 MHz, DMSO-d6) δ 8.51 (dd, J = 4.53, 1.51 Hz, 1H), 8.29 (d, J = 0.76 Hz, 1H), 8.05-8.19 (m, 1H), 7.79 (d, J = 1.13 Hz, 1H), 7.22 (dd, J = 9.44, 4.53 Hz, 1H).
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)
6775-78-6
286 suppliers
$6.00/1g
![Imidazo[1,2-b]pyridazine](/CAS/GIF/766-55-2.gif)
766-55-2
304 suppliers
$6.00/1g
Yield:766-55-2 87%
Reaction Conditions:
with palladium on activated charcoal;hydrogen;triethylamine in tetrahydrofuran;methanol for 16 h;
Steps:
21 Intermediate 21 G: Imidazo[ 1 ,2-bj pyridazine
To a stirred solution of 6-chloroimidazo[1,2-bjpyridazine (800 mg, 5.21 mmol) in methanol (20 mL) and tetrahydrofuran (20 mL) was added triethylamine (0.8 mL, 5.74mmol) followed by Pd/C (100 mg, 0.094 mmol). The mixture was stirred under hydrogen atmosphere for 16h. The reaction mixture was filtered through celite and the celite bed was washed with methanol. The combined filtrate was concentrated then suspended in water and extracted with ethyl acetate (3x). The organic layer was dried over Na2SO4 and filtered to give the imidazo[1,2-bjpyridazine (550 mg, 87%) as off white solid. LCMS[m/z 120 (M+H)j; ‘H NMR (300 MHz, DMSO-d6) ? 8.51 (dd, J4.53, 1.51 Hz, 1H) 8.29(d,J0.76Hz, 1 H) 8.05-8.19(m, 1 H)7.79(d,J1.13 Hz, 1 H) 7.22 (dd,J=9.44,4.53 Hz, 1 H).
References:
BRISTOL-MYERS SQUIBB COMPANY;DUNCIA, John V.;GARDNER, Daniel S.;HYNES, John;MACOR, John E.;MURUGESAN, Natessan;SANTELLA, Joseph B.;WU, Hong;KANTHETI, Durgarao;NAIR, Satheesh Kesavan;PAIDI, Venkatram Reddy;RATNA KUMAR, Sreekantha;SARKUNAM, Kandhasamy;SISTLA, Ramesh Kumar;POLIMERA, Subba Rao WO2016/210036, 2016, A1 Location in patent:Page/Page column 106; 107
![6-Hydrazinoimidazo[1,2-b]pyridazine](/CAS/GIF/6653-91-4.gif)
6653-91-4
23 suppliers
inquiry
![Imidazo[1,2-b]pyridazine](/CAS/GIF/766-55-2.gif)
766-55-2
304 suppliers
$6.00/1g