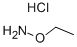
Ethoxyamine hydrochloride synthesis
- Product Name:Ethoxyamine hydrochloride
- CAS Number:3332-29-4
- Molecular formula:C2H8ClNO
- Molecular Weight:97.54

1914-21-2
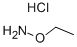
3332-29-4
1. 500 mg of hydroxyphthalimide (Aldrich) was dissolved in 5 mL of dimethylformamide (DMF) under argon protection. Subsequently, 0.27 mL of ethyl iodide (Aldrich) and 0.5 mL of 1,8-diazabicyclo[5.4.0]undec-7-ene (Aldrich) were added sequentially and slowly added dropwise. 2. The reaction mixture was stirred at 60 °C for 2 h and then cooled to room temperature. The reaction was terminated by addition of 2N hydrochloric acid solution. 3. The reaction solution was diluted with 20 mL of ethyl acetate and subsequently dried over magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography using ethyl acetate/hexane (1:5) as eluent to give 518 mg of the target compound (yield: 88%). 4. The above compound was dissolved in 5 mL of dichloromethane, and 0.11 mL of methylhydrazine (TCI) was slowly added at 0 °C. The reaction solution was stirred at room temperature for 2 hours and then cooled to 0°C again. 5. The resulting solid was collected by filtration and 1 mL of 4M dioxane hydrochloride solution (Aldrich) was added to the filtrate, which was filtered and dried to give 263 mg solid (yield: 100%). 6. 10 mg of the resulting solid was dissolved with 44 mg of SAC-0906 in 1 mL of pyridine (Aldrich) under argon protection and stirred at 80 °C for 4 hours. 7. After the reaction solution was cooled to room temperature, it was acidified by adding 2N hydrochloric acid solution and extracted with 20 mL of ether. The organic phase was dried with magnesium sulfate, filtered and concentrated under reduced pressure. 8. The residue was purified by silica gel column chromatography using ethyl acetate/hexane (1:5) as eluent to afford ethoxyamine hydrochloride SAC-1013 (46 mg, yield: 96%). 1H-NMR (300 MHz, CDCl3) δ 5.91-5.76 (m, 2H), 5.34-5.32 (m, 1H), 5.28-5.25 (m, 1H), 5.14 (m, 1H), 4.24-4.10 (m, 3H), 4.04 (q, J = 20.9 Hz, 2H), 3.64-3.48 (m, 1H ), 2.42-0.60 (m, 38H).

1914-21-2
10 suppliers
inquiry
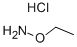
3332-29-4
288 suppliers
$5.00/5g
Yield:3332-29-4 100%
Reaction Conditions:
Stage #1: N-ethoxyphthalimidewith methylhydrazine in dichloromethane at 0 - 20; for 2 h;
Stage #2: with hydrogenchloride in 1,4-dioxane at 0;
Steps:
1-5 Preparation of SAC-1013
After 500 mg of hydroxyphthalimide (Aldrich) was dissolved in 5 ml of dimethylformamide under argon flow, 0.27 ml of iodinated ethane (Aldrich) was added, and 0.5 ml of 1,8-diazabicyclo[5.4.0]undec-7-ene (Aldrich) was slowly added. After the mixture was stirred at 60° C. for 2 hours, the temperature was again lowered to room temperature, and then the reaction was stopped by adding a 2 N hydrochloric acid solution. The reaction liquid was diluted by adding 20 ml of ethyl acetate, followed by drying over magnesium sulfate and then filtration. The filtrate was concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography using a mixture eluent of ethyl acetate/hexane (1:5) and then dried to obtain 518 mg of a compound (yield: 88%). The compound was dissolved in 5 ml of dichloromethane, and 0.11 ml of methyl hydrazine (TCI) was slowly added at 0° C. After the reaction liquid was stirred at room temperature for 2 hours, the temperature was again lowered to 0° C. The generated solid was then filtered out, and 1 ml of a 4 M-hydrochloric acid dioxane solution (Aldrich) was added to the residual filtrate, followed by filtration and drying, to obtain 263 mg of a solid (yield: 100%). 10 mg of the obtained solid and 44 mg of SAC-0906 obtained as obtained above were dissolved in 1 ml of pyridine (Aldrich) under argon flow, followed by stirring at 80° C. for 4 hours. After the temperature was lowered to room temperature, the reaction liquid was acidified by adding a 2 N hydrochloric acid solution, followed by extraction with 20 ml of diethyl ether, drying over magnesium sulfate, and filtration. The filtrate was concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography using a mixed eluent of ethyl acetate/hexane (1:5) to obtain the target compound SAC-1013 (46 mg, yield: 96%). 1H-NMR (300 MHz, CDCl3) δ5.91-5.76 (m, 2H), 5.34-5.32 (m, 1H), 5.28-5.25 (m, 1H), 5.14 (m, 1H), 4.24-4.10 (m, 3H), 4.04 (q, J=20.9 Hz, 2H), 3.64-3.48 (m, 1H), 2.42-0.60 (m, 38H)
References:
US2014/378399,2014,A1 Location in patent:Paragraph 0097
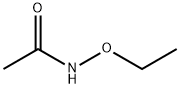
52914-24-6
1 suppliers
inquiry
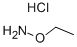
3332-29-4
288 suppliers
$5.00/5g

52176-02-0
0 suppliers
inquiry
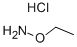
3332-29-4
288 suppliers
$5.00/5g

624-86-2
27 suppliers
inquiry
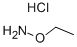
3332-29-4
288 suppliers
$5.00/5g
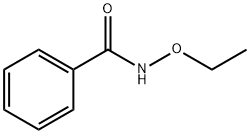
22509-51-9
2 suppliers
inquiry
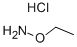
3332-29-4
288 suppliers
$5.00/5g