
1-(2-Fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione synthesis
- Product Name:1-(2-Fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione
- CAS Number:830346-47-9
- Molecular formula:C13H10F4N2O2
- Molecular Weight:302.22

830346-46-8

1694-31-1

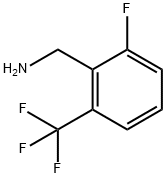
830346-47-9
Using 1-(2-fluoro-6-(trifluoromethyl)benzyl)urea (2.568 g, 10.9 mmol) and tert-butyl acetoacetate (5.0 g) in toluene (125 mL), the mixture was briefly heated to reflux using a Dean-Stark splitter. Subsequently, tert-butyl acetoacetate (5.0 g) was added and heating to reflux was continued for 4 hours. Next, p-toluenesulfonic acid monohydrate (2.82 g, 14.8 mmol) was added and reflux was continued for 1 hour. Upon completion of the reaction, toluene was replaced with isopropanol (i-PrOH) and the volume of the solution was concentrated to approximately 30 mL. The solution was stirred overnight at room temperature to crystallize the product. The crystallized product was collected by filtration and washed with a small amount of isopropanol to give 2.01 g of 1-(2-fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione in 63% yield. The product was analyzed by LCMS (ESI) at m/z 303.0 (MH+).

626-48-2
395 suppliers
$10.00/25 g
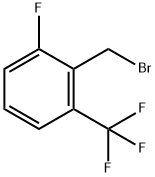
239087-08-2
210 suppliers
$14.00/1g

830346-47-9
192 suppliers
$24.00/100mg
Yield:830346-47-9 89%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide at 70; for 18 h;Solvent;Reagent/catalyst;Temperature;
Steps:
1-6 Example 1
In the reactor, 1.0 g, 1.0 equiv. as shown in Formula IV2-(Bromomethyl)-1-fluoro-3-(trifluoromethyl)benzene was dissolved in 10 mL of DMF.To this was added 1.2 equiv. 6-methyluracil as shown in Formula V, 1.8 equiv. potassium carbonate,0.1equiv. of TBAI, the system is heated to 70 ° C for 18h;After the reaction was completed, the reaction mixture was concentrated to dryness, and the concentrate was washed with a mixture of dichloromethane and 1N aqueous hydrochloric acid.The organic phase is separated, and the organic phase is washed successively with a saturated aqueous solution of sodium hydrogencarbonate and brine.Subsequently, it is dried over sodium sulfate and spin-dried to obtain 0.89 equiv. of the polysubstituted pyrimidine derivative as shown in Formula I.The yield was 89%.
References:
Anhui Nuoquan Pharmaceutical Co., Ltd.;Liu Haijie;Hu Zhigang;Xu Liangzhi;He Darong;Du Xiaopeng;Qian Zhujin;He Yong CN109761911, 2019, A Location in patent:Paragraph 0024-0037
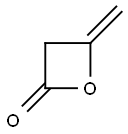
674-82-8
210 suppliers
inquiry

830346-46-8
141 suppliers
$24.00/100mg

830346-47-9
192 suppliers
$24.00/100mg

626-48-2
395 suppliers
$10.00/25 g
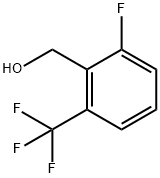
152211-15-9
96 suppliers
$20.00/1g

830346-47-9
192 suppliers
$24.00/100mg

830346-46-8
141 suppliers
$24.00/100mg
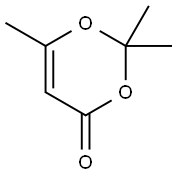
5394-63-8
230 suppliers
$19.00/25g

830346-47-9
192 suppliers
$24.00/100mg
239087-06-0
181 suppliers
$14.00/100mg

91114-97-5
10 suppliers
inquiry

830346-47-9
192 suppliers
$24.00/100mg