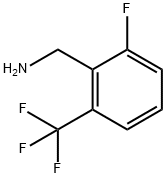
2-FLUORO-6-(TRIFLUOROMETHYL)BENZYLAMINE synthesis
- Product Name:2-FLUORO-6-(TRIFLUOROMETHYL)BENZYLAMINE
- CAS Number:239087-06-0
- Molecular formula:C8H7F4N
- Molecular Weight:193.14
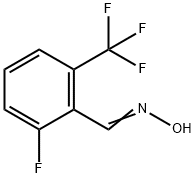
581072-15-3

239087-06-0
In a 5L three-neck flask, Intermediate B (207.0 g, 1.0 mol) was added. Ethanol (500 mL), water (200 mL) and concentrated hydrochloric acid (400 mL) were added sequentially to the reaction system and stirred for 0.5 h. The reaction mixture was cooled to 0-5 °C. Zinc powder (390 g, 6.0 mol) was slowly added while maintaining the temperature at 0-5 °C, and the rate of addition was controlled to ensure that the reaction temperature did not exceed 5 °C. After the addition of zinc powder, the reaction system was warmed up to 80-85 °C and the reaction was maintained at this temperature for 1 hour. Upon completion of the reaction, the mixture was cooled to room temperature, filtered and the filter cake was washed with ethanol (300 mL). The filtrates were combined and concentrated under reduced pressure until a significant amount of solid precipitated. The solid was collected by filtration and dried to give the target product 2-fluoro-6-(trifluoromethyl)benzylamine (169.5 g, 87.8% yield).
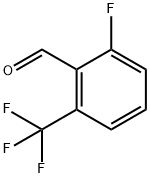
60611-24-7
202 suppliers
$10.00/1g

239087-06-0
181 suppliers
$14.00/100mg
Yield:239087-06-0 95%
Reaction Conditions:
Stage #1:2-fluoro-6-(trifluoromethyl)benzaldehyde with hydroxylamine hydrochloride;sodium hydroxide in ethanol;water for 1 h;
Stage #2: with hydrogenchloride;zinc in ethanol;water for 2 h;
Steps:
11-12 Example 12Preparation of 2-fluoro-6-trifluoromethylbenzylamine E6
Add 250 g of hydroxylamine hydrochloride and 50 ml of water to a 250 ml three-necked bottle.The aqueous phase was adjusted to neutrality with 10% sodium hydroxide, and then the ethanol solution of E5 (5g / 20ml) was slowly added dropwise. After stirring for 1h, 6g of zinc powder was added in batches.Hydrochloric acid (6 mol / L, 40 ml) was added dropwise. After 2 hours of reaction, the mixture was filtered, and 50 ml of ammonia was added to the filtrate.Adjust the pH to 11 with 6 ml of 33% sodium hydroxide, and extract with dichloromethane.The organic phase was collected, washed with saturated brine, and dried over anhydrous sodium sulfate.Suctioned and concentrated to give 4.78 g of yellow oil(95% yield).
References:
Shanghai Pharmaceutical Industry Institute;China Pharmaceutical Industry Zongyuan;Liu Yanming;Hao Qun;Lin Kuaile;Zhou Weicheng;Pan Jing;Chen Liang;Zhou Ting CN110498771, 2019, A Location in patent:Paragraph 0133-0136
133116-83-3
218 suppliers
$8.00/1g

239087-06-0
181 suppliers
$14.00/100mg

581072-15-3
6 suppliers
inquiry

239087-06-0
181 suppliers
$14.00/100mg
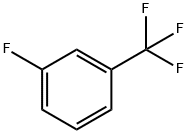
401-80-9
313 suppliers
$5.00/10g

239087-06-0
181 suppliers
$14.00/100mg
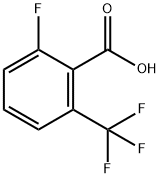
32890-94-1
147 suppliers
$12.00/1g

239087-06-0
181 suppliers
$14.00/100mg