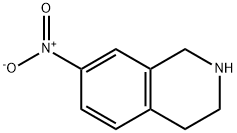
7-NITRO-1,2,3,4-TETRAHYDRO-ISOQUINOLINE synthesis
- Product Name:7-NITRO-1,2,3,4-TETRAHYDRO-ISOQUINOLINE
- CAS Number:42923-79-5
- Molecular formula:C9H10N2O2
- Molecular Weight:178.19

91-21-4

42923-79-5
In a mortar, 2 g of P2O5/silica gel (65% w/w) was mixed with 1,2,3,4-tetrahydroisoquinoline (10 mmol, 1.33 g) and milled for 30 s. Subsequently, 5 ml of 65% HNO3 was added slowly dropwise, and milling was continued for 20 min at room temperature until the mixture took on a deep yellow color. The progress of the reaction was monitored by TLC (n-hexane:EtOAc 70:30) to confirm the complete disappearance of 1,2,3,4-tetrahydroisoquinoline (about 30 min). After completion of the reaction, ether (50 ml) was added to the mixture and the solids were separated by filtration through a short silica gel pad and washed with ether (2 x 15 ml). The combined organic phases were washed with 10% NaHCO3 solution (20 ml) and subsequently dried with MgSO4. The solvent was removed by concentration under reduced pressure and the residue was purified by column chromatography (n-hexane:EtOAc, 2:1) to afford 7-nitro-1,2,3,4-tetrahydroisoquinoline (2b) (8 mmol, 1.4 g, 80% yield) as a yellow solid with a melting point of 121 °C. 1H NMR (δ): 8.05 (m, 2H), 7.60 (m, 1H), 3.82 (s, 2H), 3.38 (t, 2H, J = 7.4 Hz), 3.12 (t, 2H, J = 7.4 Hz), 2.83 (s, 1H).13C NMR (δ): 150.6, 145.0, 140.3, 129.6, 122.6, 121.4, 46.9, 44.1, 28.1.High-resolution mass spectra (EMS) [M+H]+ C9H10N2O2, calculated value 179.0740, measured value 179.1121.

91-21-4
260 suppliers
$5.00/5g

42923-79-5
125 suppliers
$15.00/250mg
Yield:42923-79-5 86%
Reaction Conditions:
with sodium nitrate;sulfuric acid at 4 - 20; for 16 h;
Steps:
5 2-Allyl- 1 -(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-((2-methyl- 1,2,3 ,4-tetrahydroisoquinolin-7-yl)amino)- 1 ,2-dihydro-3H-pyrazolo[3 ,4-d]pyrimidin-3-one
To concentrated H2504 solution (62 mL) at 4 °C was added 1,2,3,4- tetrahydroisoquinoline (15 g, 112.78 mmol) dropwise keeping the temperature below 15 °C. To the stifling mixture at 4 °C was added NaNO3 (12.5, 148.80 mmol) keeping internal temperature below 10 °C. The reaction mixture was stined at RT for 16 h. The reaction mixture was added to NH4OH (185 mL) to a final pH = 8. The mixture was extracted with DCM (3 x 150 mL), washed with brine (75 mL) and dried (Na2504). The solvent was removed. The resulting crude was dissolved in EtOH (50 mL) and concentrated HC1 (14 mL) was added. The resulting solid was filtered and washed with diethyl ether to afford 7-nitro- 1,2,3,4-tetrahydroisoquinoline (13 g, 86%) as a mixture of isomers. MS (ESI) mlz: 179.1 [M+H].
References:
WO2019/28008,2019,A1 Location in patent:Paragraph 0141

181514-37-4
5 suppliers
inquiry

42923-79-5
125 suppliers
$15.00/250mg

91-21-4
260 suppliers
$5.00/5g

42923-79-5
125 suppliers
$15.00/250mg

186390-77-2
55 suppliers
inquiry
![Acetamide, 2,2,2-trifluoro-N-[2-(4-nitrophenyl)ethyl]-](/CAS/20200515/GIF/24954-63-0.gif)
24954-63-0
1 suppliers
inquiry

42923-79-5
125 suppliers
$15.00/250mg
64-04-0
0 suppliers
$10.00/5mL

42923-79-5
125 suppliers
$15.00/250mg