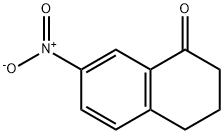
7-Nitro-1-tetralone synthesis
- Product Name:7-Nitro-1-tetralone
- CAS Number:40353-34-2
- Molecular formula:C10H9NO3
- Molecular Weight:191.18
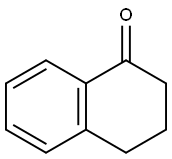
529-34-0

40353-34-2
General procedure for the synthesis of 7-nitro-3,4-dihydro-2H-1-naphthalenone from 1-tetralone: Concentrated sulfuric acid (60 mL) was cooled to 0 °C in an ice bath. 1-Tetrahydronaphthalenone (8 g, 54.7 mmol) was added with stirring, and then potassium nitrate (6 g, 59.3 mmol, 1.08 eq.) dissolved in concentrated sulfuric acid (18 mL) was slowly added dropwise through a dropping funnel, controlling the reaction temperature to no more than 15 °C. The reaction was carried out at a controlled temperature of 15 °C. The reaction was completed with the addition of potassium nitrate (6 g, 59.3 mmol, 1.08 eq.). After the dropwise addition was completed, the reaction mixture was continued to be stirred for 1 hour. The reaction was quenched by pouring the reaction solution into crushed ice, the precipitate was collected by filtration and washed with distilled water. After drying, recrystallization was carried out through a solvent mixture of ethanol/water (1:1) to give 7-nitro-3,4-dihydro-2H-1-naphthalenone (8.5 g, 81% yield) as a light yellow solid with a melting point of 104-106 °C. The product was analyzed by infrared spectroscopy (IR, film) showing characteristic absorption peaks located at 1675 cm?1, 1500 cm?1 and 1340 cm?1. The nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 400 MHz, CDCl3) data are as follows: δ 2.18-2.25 (2H, m, CH2), 2.75 (2H, t, J = 6.8 Hz, CH2), and 3.10 (2H, t, J = 6.1 Hz, CH2), 7.45 (1H, d, J = 8.4 Hz, ArH), 8.30 (1H, dd, J = 2.4 Hz, 8.4 Hz, ArH), 8.86 (1H, d, J = 2.4 Hz, ArH).

529-34-0
485 suppliers
$6.00/10g

40353-34-2
133 suppliers
$8.00/250mg
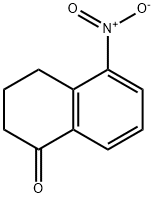
51114-73-9
87 suppliers
$13.00/250mg
Yield:51114-73-9 16% ,40353-34-2 77%
Reaction Conditions:
with sulfuric acid;nitric acid at 0; for 1 h;
Steps:
125
Λ/-(5,6,7,8-Tetrahydro-2-naphthalenyl)acetamide (166). fHNO3 (8.6 mL, 144 mmol) in CH2SO4 (50 mL) was added dropwise to a stirred solution of α-tetralone (165) (20 g, 137 mmol) in CH2SO4 (300 mL) at 0 0C and the solution stirred for 1 h. The solution was poured into ice/water (2 L), stirred for 30 min, filtered and washed with water. The solid was dried and purified by chromatography, eluting with 20% EtOAc/pet. ether, to give (i) 5-nitro-3,4-dihydro-1(2H)-naphthalenone (4.1 g, 16%) as a white solid: 1H NMR δ 8.35 (dd, J = 7.8, 1.4 Hz, 1 H, H-6), 8.09 (dd, J = 8.0, 1.4 Hz, 1 H, H-8), 7.48 (br t, J = 7.9 Hz, 1 H, H-7), 3.22 (t, J = 6.1 Hz, 2 H, H-4), 2.74 (dd, J = 6.8, 6.4 Hz, 2 H, H-2), 2.13-2.21 (m, 2 H, H-3); and (ii) 7-nitro-3,4-dihydro-1(2H)-naphthalenone (20.1 g, 77%) as a white solid: 1H NMR δ 8.86 (d, J = 2.5 Hz, 1 H, H-4), 8.30 (dd, J = 8.4, 2.5 Hz, 1 H, H-6), 7.46 (d, J = 8.4 Hz, 1 H, H-5), 3.09 (t, J = 6.1 Hz, 2 H, H-4), 2.74 (dd, J = 7.0, 6.2 Hz, 2 H, H-2), 2.17- 2.25 (m, 2 H, H-3).A solution of 7-nitro-3,4-dihydro-1 (2H)-naphthalenone (1.67 g, 8.7 mmol) in EtOAc/EtOH (1 :1, 150 mL), water (15 mL) and cHCI (2 mL) with Pd/C (5%, 500 mg) was stirred vigorously under H2 (60 psi) for 16 h. The suspension was filtered through Celite, washed with EtOH (4 x 10 mL) and the organic solvent evaporated. The aqueous residue was partitioned between DCM and dilute aqueous NH3 solution and the organic fraction dried and the solvent evaporated. The residue was dissolved in dioxane (20 mL), and Ac2O (1.8 mL, 19.2 mmol) was added dropwise to the solution at 0 0C. The solution was stirred at 20 0C for 16 h, diluted with water (50 mL), and partitioned between EtOAc and dilute aqueous NH3 solution. The organic fraction was washed with water (3 20 mL), dried and the solvent evaporated to give Λ/-(5,6,7,8-tetrahydro-2-naphthalenyl)acetamide 166 (1.57 g, 95%) as a white solid: 1H NMR δ 7.18-7.25 (m, 2 H, H-1, NH), 7.15 (dd, J = 8.2, 2.1 Hz, 1 H, H-3), 7.00 (d, J = 8.2 Hz, 1 H, H-4), 2.69-2.77 (m, 4 H, 2 x CH2), 2.15 (s, 3 H, CH3), 1.74-1.80 (m, 4 H, 2 x CH2). The procedure was repeated a number of times to give N- (5,6,7, 8-tetrahydro-2-naphthalenyl)acetamide 166 (10.21 g, 88% overall).
References:
AUCKLAND UNISERVICES LIMITED WO2006/104406, 2006, A1 Location in patent:Page/Page column 126

529-34-0
485 suppliers
$6.00/10g

40353-34-2
133 suppliers
$8.00/250mg
89781-52-2
50 suppliers
$213.00/1g

40353-34-2
133 suppliers
$8.00/250mg

529-34-0
485 suppliers
$6.00/10g

40353-34-2
133 suppliers
$8.00/250mg
1821-12-1
427 suppliers
$6.00/5g

40353-34-2
133 suppliers
$8.00/250mg