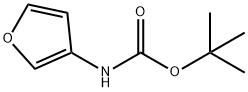
Carbamic acid, 3-furanyl-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, 3-furanyl-, 1,1-dimethylethyl ester (9CI)
- CAS Number:56267-48-2
- Molecular formula:C9H13NO3
- Molecular Weight:183.2
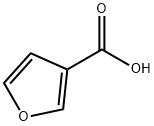
488-93-7
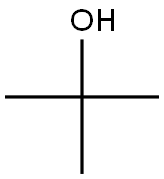
75-65-0

56267-48-2
General procedure for the synthesis of tert-butyl furan-3-yl carbamate from 3-furoic acid and tert-butanol: To a solution of toluene (800 mL) containing 3-furoic acid (54.4 g, 485 mmol), triethylamine (105 mL, 753 mmol), and tert-butanol (25.2 mL, 786 mmol) was added slowly and dropwise at 45 °C at room temperature diphenylphosphoryl azide (157.8 mL, 732 mmol) at 45 °C. After dropwise addition, the reaction mixture was heated to reflux for 6 hours, followed by stirring at room temperature overnight. Upon completion of the reaction, the reaction mixture was diluted with water (1000 mL) and extracted twice with ethyl acetate (1000 mL). The organic layers were combined, washed sequentially with water (800 mL) and brine (800 mL), decolorized by activated charcoal, dried, filtered and concentrated in vacuum to give a brown semi-solid product. The semi-solid product was crystallized by a solvent mixture of dichloromethane (300 mL) and hexane (600 mL) to finally obtain tert-butyl furan-3-yl carbamate (61.5 g, 78% yield). The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 7.71 (s, 1H), 7.30-7.24 (m, 1H), 6.43 (s, 1H), 6.27 (s, 1H), 1.75-1.32 (s, 9H).

488-93-7
397 suppliers
$5.00/250mg

75-65-0
785 suppliers
$10.00/10ml

56267-48-2
142 suppliers
$16.00/100mg
Yield:56267-48-2 78%
Reaction Conditions:
with diphenyl phosphoryl azide;triethylamine in toluene at 20;Reflux;
Steps:
2.e
e. To a solution of 3-furoic acid 96 (54.4 g, 485 mmol), triethylamine (105 ml, 753 mmol), tert-butanol (25.2 mL, 786 mmol) in toluene (800 mL) was added dropwise at room temperature over 45 min period diphenyl phosphoryl azide (157.8 mL, 732 mmol). The resulting solution was heated at reflux for 6 h and at room temperature overnight. The reaction was diluted with water (1000 mL) and extracted twice with ethyl acetate (1000 ml). The organic layers were combined washed with water (800 mL), brine (800 mL), decolorized with activated charcoal, dried, filtered and concentrated in vacuo to furnish a brown semisolid. The semisolid was crystallized from dichloromethane (300 mL) and hexanes (600 mL) to furnish tert-butyl furan-3-ylcarbamate 97 (61.5 g, 78%). 1H NMR (300 MHz, CDC13) δ 7.71 (s, IH), 7.30 - 7.24 (m, IH), 6.43 (s, IH), 6.27 (s, IH), 1.75 - 1.32 (s, 9H).
References:
BIOCRYST PHARMACEUTICALS, INC.;BABU, Yarlagadda, S.;CHAND, Pooran;KOTIAN, Pravin, L.;KUMAR, V., Satish WO2010/14930, 2010, A2 Location in patent:Page/Page column 39

22037-28-1
339 suppliers
$24.00/5g
4248-19-5
378 suppliers
$5.00/5g

56267-48-2
142 suppliers
$16.00/100mg