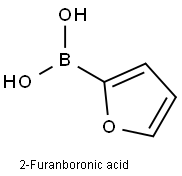
2-Furanboronic acid synthesis
- Product Name:2-Furanboronic acid
- CAS Number:13331-23-2
- Molecular formula:C4H5BO3
- Molecular Weight:111.89

1104637-62-8

13331-23-2
The general procedure for the synthesis of 2-furanboronic acid from 2-(furan-2-yl)-6-methyl-1,3,6,2-dioxaborolane-4,8-dione was as follows: a solution of MIDA borate (5 mmol) in THF (50 mL) was added to a 100 mL flask fitted with a stir bar under an ambient atmosphere followed by the addition of an aqueous 1.0 M NaOH solution (15 mL ). The reaction mixture was stirred vigorously for 20 minutes. Upon completion, the mixture was transferred to a partition funnel and diluted with ether (50 mL) and 0.5 M pH 7 sodium phosphate buffer (50 mL). The phases were separated after sufficient shaking. The aqueous phase was extracted with a solvent mixture of THF/ether (1:1, 2 x 25 mL). The organic phases were combined, dried with anhydrous MgSO4, filtered and concentrated in vacuum. The residual solvent was co-evaporated with acetonitrile and the resulting solid was dried under vacuum at about 1 Torr for 30 min. The resulting boric acid was analyzed by 1H-NMR to be >95% pure and was immediately used in the subsequent cross coupling reaction. Specific examples are as follows: 2-furanboronic acid (0.531 g, 95% yield) was obtained as an off-white solid using MIDA borate (1.127 g, 5.002 mmol) following the general procedure described above.TLC (unfolding reagent: ethyl acetate) Rf = 0.46, stained with potassium permanganate to develop color.1H-NMR (500 MHz, DMSO-d6:D2O 1H-NMR (500 MHz, DMSO-d6:D2O 95:5, with TMS) δ 7.81 (dd, J = 1.5, 5.5 Hz, 1H), 7.07 (dd, J = 3.0, 0.5 Hz, 1H), 6.48 (dd, J = 3.5, 2.0 Hz, 1H). 13C-NMR (125 MHz, DMSO-d6:D2O 95:5, with TMS) δ 146.4, 121.5, 110.3. 121.5, 110.3. HRMS (EI+) calculated value C4H3O3B [M]+: 112.0332, measured value: 112.0332.

1104637-62-8
13 suppliers
$44.50/1g

13331-23-2
346 suppliers
$13.43/250mgs:
Yield:13331-23-2 95%
Reaction Conditions:
Stage #1:2-(furan-2-yl)-6-methyl-1,3,6,2-dioxazaborocane-4,8-dione with water;sodium hydroxide in tetrahydrofuran at 23; for 0.333333 h;
Stage #2: in tetrahydrofuran;diethyl ether; pH=7Aqueous phosphate buffer;
Steps:
5
The general method for synthesizing unprotected organoboronic acids was as follows. Under ambient atmosphere, to a 100 mL flask equipped with a stir bar and charged with MIDA boronate (2) (5 mmol) as a solution in THF (50 mL) was added aqueous NaOH (1.0 M, 15 mL). The mixture was vigorously stirred for 20 min. The mixture was then transferred to a separatory funnel and was diluted with EbO (50 mL) and 0.5 M pH 7 sodium phosphate buffer (50 mL). The mixture was shaken, and the phases were separated. The aqueous phase was extracted with THFiEt2O (1 :1 , 2 x 25 mL). The combined organics were dried over MgSO4, filtered and concentrated in vacuo. Residual solvent was co-evaporated with MeCN, and the resulting solid was placed under vacuum ( ~ 1 Torr) for 30 min. All boronic acids thus obtained were judged to be > 95% pure by 1 H-NMR and were utilized in cross-coupling reactions immediately after preparation. An example of this method is depicted in the scheme below. To form unprotected organoboronic acid 1a, the general procedure was followed using MIDA boronate 2a (1.127 g, 5.002 mmol) to yield the 1a as an off white solid (0.531 g, 95%). TLC (EtOAc) Rf = 0.46, stained with KMnO4. 1H-NMR (500 MHz, DMSO-d6:D2O 95:5 w/ TMS) δ 7.81 (dd, / = 1 .5, 0.5 Hz, 1 H), 7.07 (dd, / = 3.0, 0.5 Hz, 1 H), 6.48 (dd, / = 3.5, 2.0 Hz, 1 H). 13C-NMR (125 MHz, DMSO-d6:D2O 95:5 w/ TMS) δ 146.4, 121.5, 1 10.3. HRMS (El +) Calculated for C4HsO3B (M) + : 1 12.0332, Found: 1 12.0332.
References:
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS;BURKE, Martin, D.;KNAPP, David, M.;GILLIS, Eric, P. WO2010/36921, 2010, A2 Location in patent:Page/Page column 41

534-22-5
310 suppliers
$10.00/5mL
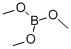
121-43-7
384 suppliers
$13.00/25mL

13331-23-2
346 suppliers
$13.43/250mgs:

110-00-9
224 suppliers
$10.00/25g
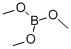
121-43-7
384 suppliers
$13.00/25mL

7732-18-5
498 suppliers
$12.69/100ml

13331-23-2
346 suppliers
$13.43/250mgs:
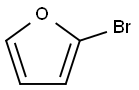
584-12-3
177 suppliers
$20.00/1g
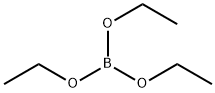
150-46-9
204 suppliers
$12.00/25mL

13331-23-2
346 suppliers
$13.43/250mgs:

3187-94-8
9 suppliers
inquiry

13331-23-2
346 suppliers
$13.43/250mgs: