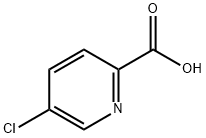
5-Chloropyridine-2-carboxylic acid synthesis
- Product Name:5-Chloropyridine-2-carboxylic acid
- CAS Number:86873-60-1
- Molecular formula:C6H4ClNO2
- Molecular Weight:157.55
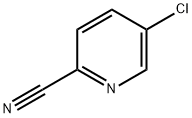
89809-64-3

86873-60-1
The general procedure for the synthesis of 5-chloro-2-cyanopyridine-2-carboxylic acid from 5-chloro-2-cyanopyridine was as follows: in a 1000 mL four-necked flask equipped with a stirrer, a thermometer, and a reflux condenser, 5-chloro-2-cyanopyridine (27.7 g, 0.2 mol) and 277 mg of ethanol were added, and the mixture was stirred until it was completely dissolved to form an aqueous solution. Subsequently, 277 mL of 10% NaOH solution was added and the mixture was heated to 90-100 °C and stirred under reflux conditions for 1.5 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) [unfolding reagent ratio of butanol-acetic acid-water = 5:2:2], and after confirming the completion of the reaction, the reaction solution was stirred and cooled to room temperature. The pH was adjusted to 2 with 2 N HCl solution and subsequently concentrated under reduced pressure to about 100 mL. Methanol was added to the concentrate to a total volume of 200 mL, and the solid product was collected by filtration after cooling to 0-5 °C. The filtrate was then filtered. The filtrate was concentrated to dryness under reduced pressure and the residue was dissolved in a solvent mixture of 450 mL of dichloromethane and 50 mL of methanol, stirred for 1 hr and then filtered, and the resulting solid was washed with a 10% methanol-dichloromethane solvent mixture. Finally, the solid was dried in a vacuum oven at 60~65°C for 2 h. The white solid intermediate 5-chloropyridine-2-carboxylic acid was obtained as 25.5 g in 81.4% yield.

89809-64-3
267 suppliers
$14.00/5g

86873-60-1
250 suppliers
$7.00/1g
Yield:86873-60-1 81.4%
Reaction Conditions:
with water;sodium hydroxide in ethanol at 90 - 100; for 1.5 h;
Steps:
1.5 5) Preparation of Intermediate 5
1000ml four-necked flask, fitted with a stirrer, thermometer, reflux condenser, is added 5-chloro-2-cyano-pyridine (27.7g, 0.2mo), 277 mg of the ethanol, and dissolved with stirring, an aqueous solution of 10% NaOH was added 277ml, heated to 90 ~ 100 stirred at reflux for 1.5h, TLC TLC [discriminating the end of reaction conditions to expand (butanol - acetic acid - water = 5: 2: 2)], the reaction is complete, cooled to room temperature under stirring, the pH was adjusted to 2 with 2NHCl ~ 3, and concentrated under reduced pressure to a volume of 100ml, methanol was added to 200ml, cooled to 0 ~ 5 , filtered, the solid out, and the filtrate was concentrated to dryness under reduced pressure, the residue was added 450ml of dichloromethane and 50ml of methanol, stirred for 1h, filtered and the solid with the amount of 10% methanol - methylene chloride mixture was washed, 60 ~ 65 vacuum oven vacuum drying 2h, as a white solid intermediate 525.5g, a yield of 81.4%.
References:
Anhui Yi Xin Ming Pharmaceutical Co., Ltd.;Xu, Hui;Tao, Junyu CN105348187, 2016, A Location in patent:Paragraph 0014; 0032

40473-01-6
431 suppliers
$5.00/1g
124-38-9
132 suppliers
$175.00/23402

86873-60-1
250 suppliers
$7.00/1g
1072-98-6
716 suppliers
$5.00/1g

86873-60-1
250 suppliers
$7.00/1g

223445-06-5
18 suppliers
inquiry

86873-60-1
250 suppliers
$7.00/1g
2138-22-9
133 suppliers
$24.00/1g

86873-60-1
250 suppliers
$7.00/1g