
2-Bromo-5-chloropyridine synthesis
- Product Name:2-Bromo-5-chloropyridine
- CAS Number:40473-01-6
- Molecular formula:C5H3BrClN
- Molecular Weight:192.44
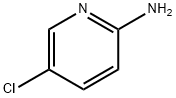
1072-98-6

40473-01-6
General procedure for the synthesis of 2-bromo-5-chloropyridine from 2-amino-5-chloropyridine: [Ref. Example 25] 5-(5-chloro-2-pyridinyl)-1-(3-pyridinyl)-1H-pyrazole-3-carboxylic acid; [show image] 1) Hydrogen bromide (12 ml, 47% aqueous solution) was slowly added to hydrogen bromide (12 ml, 47% aqueous solution) at 0 °C in a hydrobromic acid containing 2-amino-5-chloropyridine (5 g) ( 50 ml, 47%) solution. Subsequently, a solution of sodium nitrite (15 g) dissolved in water (20 ml) was added dropwise to the reaction mixture. The reaction solution was stirred at 0°C for 1 h. After that, a solution of sodium hydroxide (32 g) in water (80 ml) and ethyl acetate was added for extraction. The organic and aqueous phases were separated and the organic phase was dried over anhydrous sodium sulfate. After filtration, the solvent was removed by concentration under reduced pressure to give 2-bromo-5-chloropyridine (6.8 g, 91% yield) as a white solid. The product was characterized by 1H-NMR (400 MHz, CDCl3): δ 7.44 (1H, d, J = 8.42 Hz), 7.54 (1H, m), 8.36 (1H, s).

1072-98-6
716 suppliers
$5.00/1g

40473-01-6
431 suppliers
$5.00/1g
Yield:40473-01-6 93%
Reaction Conditions:
with hydrogen bromide;bromine;sodium nitrite in water at 0 - 10; for 1.5 h;
Steps:
2-Bromo-5-chloropyridine (3).
Bromine (26 ml, 0.52 mol)was added to a solution of 2-amino-5-chloropyridine (2)(25.6 g, 0.2 mol) in 48% HBr solution (100 ml, 1.2 mol),while maintaining the temperature at solution of sodium nitrite (32.4 g, 0.47 mol) in water(50 ml) was added over 1 h at the same temperature. Afterthe addition was complete, the reaction mixture was stirredfor additional 30 min and then treated with a solution ofNaOH (74.6 g, 1.86 mol) in water (100 ml) at such a ratethat the temperature did not exceed 20-25°C. The obtainedprecipitate was filtered off, washed with a saturatedsolution of NaHSO3 (~5 ml), several times with ice water(3×30 ml), and air-dried. Yield 35.6 g (93%), beige powder,mp 67-68° (hexane) (mp 68-69°10). Rf -0.4 (CHCl3).1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.44 (1H, d,J = 8.4, H-3); 7.54 (1, dd, J = 8.4, J = 2.6, H-4); 8.35(1H, d, J = 2.6, H-6). 13C NMR spectrum (CDCl3), δ, ppm:128.7; 131.5; 138.1; 139.2; 148.6.
References:
Alekseyev, Roman S.;Amirova, Sabina R.;Terenin, Vladimir I. [Chemistry of Heterocyclic Compounds,2017,vol. 53,# 2,p. 196 - 206][Khim. Geterotsikl. Soedin.,2017,vol. 53,# 2,p. 196 - 206,11]

1072-98-6
716 suppliers
$5.00/1g

40473-01-6
431 suppliers
$5.00/1g
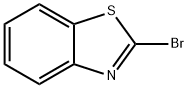
2516-40-7
186 suppliers
$10.00/1g
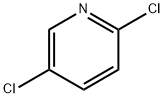
16110-09-1
466 suppliers
$5.00/1g

40473-01-6
431 suppliers
$5.00/1g
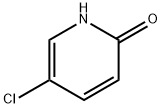
4214-79-3
248 suppliers
$6.00/1g

40473-01-6
431 suppliers
$5.00/1g