
5-BROMOPENTANOIC ACID, T-BUTYL ESTER synthesis
- Product Name:5-BROMOPENTANOIC ACID, T-BUTYL ESTER
- CAS Number:88987-42-2
- Molecular formula:C9H17BrO2
- Molecular Weight:237.13

2067-33-6
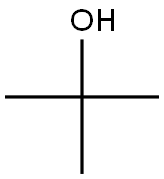
75-65-0

88987-42-2
5-Bromovaleric acid (5 g, 27.62 mmol) was dissolved in anhydrous dichloromethane (DCM), and the solution was brought to room temperature and stirred under cooling in an ice bath. N,N'-dimethylaminopyridine (DMAP, 1.68 g, 13.75 mmol) and dicyclohexylcarbodiimide (DCC, 6.83 g, 33.15 mmol) were added sequentially. After half an hour of reaction, tert-butanol (15.8 ml, 165.75 mmol) was added to the mixture. The reaction solution was stirred at room temperature overnight. After completion of the reaction, the reaction mixture was diluted with dichloromethane and washed sequentially with water and brine. The organic layer was separated, dried over anhydrous sodium sulfate and evaporated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography with hexane-ethyl acetate (1%) as eluent to give pure tert-butyl 5-bromopentanoate 4.1 g (62.6% yield) as a light yellow liquid.
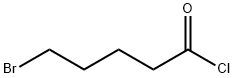
4509-90-4
236 suppliers
$15.00/1g

75-65-0
785 suppliers
$10.00/10ml

88987-42-2
84 suppliers
$9.00/100mg
Yield: 3.02 g
Reaction Conditions:
with oxalyl dichloride in dichloromethane at 0 - 20;
Steps:
1.ED.3 Step 3: tert-Butyl 5-bromopentanoate
To a solution of 5-bromovaleric acid (2.5 g, 14.0 mmol) in dichloromethane (10 mL) was added oxalyl chloride (1.15 mL, 13.2 mmol) and the reaction mixture stirred at room temperature for 4 hours. The solvent and excess oxalyl chloride were removed under vacuum. The crude acid chloride was dissolved in dichloromethane (10 mL) and cooled to 0°C. Then fert-butanol (10 mL) was added and the reaction mixture stirred at room temperature overnight. The solvent was removed, and the crude was purified by flash chromatography (SiCh: ethyl acetate/hexane 0 to 50%) to get fert-butyl 5-bromopentanoate (3.02 g, 92%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ: 3.40 (t, 2H, J = 6.6 Hz); 2.24 (t, 2H, J = 7.3 Hz); 1.90-1.81 (m, 2H); 1.75-1.69 (m, 2H); 1.43 (s, 9H).
References:
MODERNATX, INC.;BENENATO, Kerry E.;BUTCHER, William WO2018/232120, 2018, A1 Location in patent:Paragraph 001036

2067-33-6
403 suppliers
$6.00/5g

75-65-0
785 suppliers
$10.00/10ml

88987-42-2
84 suppliers
$9.00/100mg

2067-33-6
403 suppliers
$6.00/5g
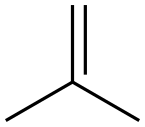
115-11-7
244 suppliers
$45.00/25 g

88987-42-2
84 suppliers
$9.00/100mg

75-65-0
785 suppliers
$10.00/10ml

88987-42-2
84 suppliers
$9.00/100mg

2067-33-6
403 suppliers
$6.00/5g
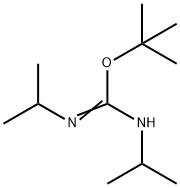
71432-55-8
149 suppliers
$6.00/250mg

88987-42-2
84 suppliers
$9.00/100mg