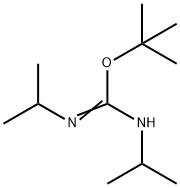
2-TERT-BUTYL-1,3-DIISOPROPYLISOUREA synthesis
- Product Name:2-TERT-BUTYL-1,3-DIISOPROPYLISOUREA
- CAS Number:71432-55-8
- Molecular formula:C11H24N2O
- Molecular Weight:200.32
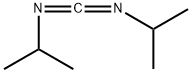
693-13-0
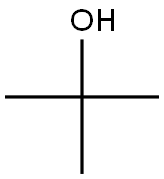
75-65-0

71432-55-8
GENERAL STEPS: CuCl (63.4 mg, 0.64 mmol, 1 mol%) was added to a solution of N,N'-diisopropylcarbodiimide (10.0 mL, 63.9 mmol, 1.0 equiv) in tert-butanol (6.97 mL, 73.5 mmol, 1.15 equiv). The reaction mixture was stirred at room temperature for 14 hours. After completion of the reaction, purification was carried out by distillation under reduced pressure (80°C, 25 mmHg). 2-tert-butyl-1,3-diisopropylisourea was obtained as a colorless oil (10.4 g, 51.9 mmol, 81% yield). The product was characterized by 1H NMR (300 MHz, CDCl3): δ 3.80-3.58 (1H, m, CH), 3.31-2.97 (1H, m, CH), 1.44 (9H, s, tBu), 1.19-1.01 (12H, m, Me2×2).

777031-92-2
0 suppliers
inquiry

75-65-0
784 suppliers
$10.00/10ml

71432-55-8
149 suppliers
$6.00/250mg
Yield:-
Reaction Conditions:
with copper(l) chlorideNeat (no solvent);Heating / reflux;
Steps:
1
Example 1 Preparation of compound 59 4-Hydroxy-pyrrolidine-1, 2-dicarboxylic acid 1-benzyl ester 2-tert-butyl ester (3) [0155] Commercially available (Bachem) Z-Hydroxy- proline (l) (10g, 42.51mmols) was dissolved 90mls of THF (tetrahydrofuran) and was cooled to 0 °C with an ice water bath. To this was added previously prepared tert- butyl N, N'-diisopropyl-imidocarbamate (2) (27ml, 135mmol) via a dropping funnel over 30 minutes. After addition the cooling bath was removed and the reaction stirred at ambient temperature for 24 hours. The volume of the reaction was reduced and then diethyl ether was added prior to washing with saturated sodium bicarbonate, then 0.5M hydrochloric acid, then water, and finally with brine. The organic layer was dried with sodium sulfate and concentrated in vacuo to give 15g of crude material. Material was run through a plug of Si02 and eluted with 45% EtOAc-Hexanes to give 4-Hydroxy-pyrrolidine-1, 2- dicarboxylic acid 1-benzyl ester 2-tert-butyl ester 3 as a colorless oil 11. Og (81%)
References:
WO2005/35525,2005,A2 Location in patent:Page/Page column 57
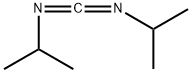
693-13-0
560 suppliers
$13.00/1ml

75-65-0
784 suppliers
$10.00/10ml

71432-55-8
149 suppliers
$6.00/250mg