
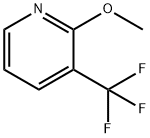
5-bromo-2-methoxy-3-(trifluoromethyl)pyridine synthesis
- Product Name:5-bromo-2-methoxy-3-(trifluoromethyl)pyridine
- CAS Number:1214377-42-0
- Molecular formula:C7H5BrF3NO
- Molecular Weight:256.02
121643-44-5

1214377-42-0
General procedure for the synthesis of 2-methoxy-3-trifluoromethyl-5-bromopyridine from 2-methoxy-3-trifluoromethylpyridine: 1. Trifluoroacetic acid (TFA, 80 mL) was added to a mixture of 2-methoxy-3-trifluoromethylpyridine (20.0 g, 113.0 mmol) and 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione (43.6 g, 152.0 mmol), and stirred for 18 hours at room temperature under argon protection. 2. Upon completion of the reaction, the TFA was removed by distillation under reduced pressure at 50 mbar and 45 °C. The residue was suspended in tert-butyl methyl ether (200 mL), filtered to remove the resulting colorless solid, and washed with tert-butyl methyl ether (50 mL). 3. The filtrate was concentrated under reduced pressure and suspended in ethyl acetate (EtOAc, 50 mL), filtered again to remove the insoluble colorless solid, and washed with ethyl acetate (50 mL). 4. The filtrate was concentrated under reduced pressure, diluted with heptane/tert-butyl methyl ether (5:1, 20 mL), and filtered to remove the insoluble colorless solid. 5. The filtrate was purified by silica gel column chromatography with heptane/ethyl acetate (100/0 to 90/10) as eluent. 6. The crude product was filtered through a plug of sodium bicarbonate (NaHCO3, 20 g) and the filtrate was evaporated under reduced pressure to give a golden yellow oil (27.9 g). 7. The oily substance was dissolved in heptane (20 mL), purified by filtration through a plug of silica gel (80 g), and eluted with heptane to afford 5-bromo-2-methoxy-3-trifluoromethylpyridine as a colorless oil (22.5 g, 74% yield). 1H-NMR (400MHz, DMSO-d6, 298K): δ 4.03 (s, 3H), 7.95 (d, 1H), 8.4 (d, 1H).

121643-44-5
226 suppliers
$5.00/1g

1214377-42-0
150 suppliers
$20.00/1g
Yield: 74%
Reaction Conditions:
Stage #1:2-methoxy-3-(trifluoromethyl)pyridine with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;trifluoroacetic acid at 20; for 18 h;Inert atmosphere;
Stage #2: with sodium hydrogencarbonate in n-heptane;ethyl acetate
Steps:
Intermediate 1 : 5-Bromo-2-methoxy-3-trifluoromethyl-pyridine To 2-methoxy-3-(trifluoromethyl)pyridine (20.0 g, 1 13.0 mmol) and 1 ,3-dibromo-5,5- dimethylimidazolidine-2,4-dione (43.6 g, 152.0 mmol) was added TFA (80 mL) and the resulting mixture stirred at rt for 18h under argon. The TFA was removed in vacuo (50 mbar, 45°C) and the residue suspended in tert-butyl methyl ether (200 mL). The resulting colourless solid was removed by filtration and washed with tert-butyl methyl ether (50 mL). The filtrate was concentrated in vacuo and suspended in EtOAc (50 mL) The insoluble colourless solid was removed by filtration and washed with EtOAc (50 mL).The filtrate was concentrated in vacuo, diluted with heptane/ tert-butyl methyl ether (5/1 , 20 mL) and the insoluble colourless solid was removed by filtration. The filtrate was purified by column chromatography on silica gel with heptane / EtOAc, 100/0 to 90/10. The crude product was filtered through a plug of NaHC03 (20g) and the filtrate evaporated in vacuo to give a golden oil (27.9 g). The oil was dissolved in heptanes (20 mL) and purified by filtered through a plug of silica gel (80 g), eluting with heptane to give 5-bromo-2-methoxy-3-(trifluoromethyl)pyridine as a colourless oil (22.5g, 74% yield). 1 H-NMR (400 MHz, DMSO-d6, 298 K): δ ppm 4.03 (s, 3H) 7.95 (d, 1 H) 8.4 (d, 1 H).
References:
NOVARTIS AG;COOKE, Nigel Graham;FERNANDES GOMES DOS SANTOS, Paulo;GRAVELEAU, Nadege;HEBACH, Christina;H?GENAUER, Klemens;HOLLINGWORTH, Gregory;SMITH, Alexander Baxter;SOLDERMANN, Nicolas;STOWASSER, Frank;STRANG, Ross;TUFILLI, Nicola;VON MATT, Anette;WOLF, Romain;ZECRI, Frédéric WO2012/4299, 2012, A1 Location in patent:Page/Page column 66-67
65753-47-1
379 suppliers
$8.00/5g

1214377-42-0
150 suppliers
$20.00/1g