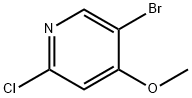
5-BROMO-2-CHLORO-4-METHOXYPYRIDINE synthesis
- Product Name:5-BROMO-2-CHLORO-4-METHOXYPYRIDINE
- CAS Number:880870-13-3
- Molecular formula:C6H5BrClNO
- Molecular Weight:222.47

17228-69-2

880870-13-3
2-Chloro-4-methoxypyridine (13.3 g, 92.6 mmol) was used as starting material and dissolved in concentrated sulfuric acid (65 mL). N-bromosuccinimide (16.5 g, 92.6 mmol) was added to the solution in batches under ice bath cooling conditions. Subsequently, the reaction mixture was stirred at 0 °C and gradually warmed up to 55 °C and kept at this temperature for 3 hours. After completion of the reaction, the mixture was slowly poured into ice water and the pH was adjusted to alkaline with 8N aqueous sodium hydroxide solution. Extraction was carried out with chloroform and the combined organic phases were washed with saturated brine and dried with anhydrous sodium sulfate. After filtration to remove the desiccant, the filtrate was concentrated under reduced pressure. The resulting crude product was purified by silica gel column chromatography with an eluent ratio of hexane:ethyl acetate (9:1 to 5:1) to afford 5-bromo-2-chloro-4-methoxypyridine as a white solid (9.3 g, 45% yield). The structure of the product was confirmed by 1H NMR (CDCl3, 300 MHz) with the following chemical shifts (δ): 3.97 (3H, s, OCH3), 6.84 (1H, s, pyridine ring H), 8.34 (1H, s, pyridine ring H).

17228-69-2
211 suppliers
$10.00/1g

880870-13-3
91 suppliers
$40.00/250mg
Yield: 45%
Reaction Conditions:
with N-Bromosuccinimide;sulfuric acid at 0 - 55; for 3 h;
Steps:
8.8
2-Chloro-4-methoxypyridine (13.3 g, 92.6 mmol) was dissolved in concentrated sulfuric acid (65 ml), N-bromosuccinimide (16.5 g, 92.6 mmol) was added under ice-cooling, and the mixture was stirred with heating at 55° C. for 3 hr. The reaction mixture was poured into ice water, alkalified with 8N aqueous sodium hydroxide solution, and extracted with chloroform. The organic layer was washed with saturated brine, and dried over sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure and the residue was purified by silica gel chromatography (hexane:ethyl acetate=9:1 to 5:1) to give the object product as a white solid (9.3 g, yield 45%). 1H NMR(CDCl3 300 MHz) (δ) ppm: 3.97 (3H, s), 6.84 (1H, s), 8.34 (1H, s)
References:
Satoh, Motohide;Aramaki, Hisateru;Nakamura, Hiroshi;Inoue, Masafumi;Kawakami, Hiroshi;Shinkai, Hisashi;Matsuzaki, Yuji;Yamataka, Kazunobu US2006/84665, 2006, A1 Location in patent:Page/Page column 49

849937-96-8
159 suppliers
$35.00/1g
124-41-4
727 suppliers
$12.00/25g

880870-13-3
91 suppliers
$40.00/250mg
