
4-BOC-1-(5-BROMO-2-PYRIDYL)PIPERAZINE synthesis
- Product Name:4-BOC-1-(5-BROMO-2-PYRIDYL)PIPERAZINE
- CAS Number:153747-97-8
- Molecular formula:C14H20BrN3O2
- Molecular Weight:342.23

624-28-2
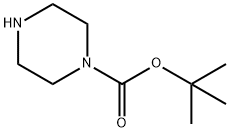
57260-71-6

153747-97-8
General procedure for the synthesis of 4-Boc-1-(5-bromo-2-pyridinyl)piperazine from 2,5-dibromopyridine and N-BOC-piperazine: 2,5-dibromopyridine (2 g, 10.7 mmol), sodium tert-butoxide (1.6 g, 16.6 mmol), xantphos (400 mg, 0.7 mmol) and toluene (100 mL) and purged with argon for 5 min. Subsequently, tert-butyl piperazine-1-carboxylate (3.4 g, 14.30 mmol) and Pd2(dba)3 (200 mg, 0.21 mmol) were added to the reaction system, and the reaction was heated at 80 °C for 4 h (TLC monitoring showed complete consumption of raw materials). Upon completion of the reaction, the reaction mixture was diluted with EtOAc (200 mL), water (100 mL) was added, filtered through a bed of diatomaceous earth, and the filter bed was washed with EtOAc (2 x 30 mL). The organic phases were combined, washed with brine (50 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (100-200 mesh, 50 g, 10-20% EtOAc-hexane gradient elution) to give tert-butyl 4-(5-bromo-2-pyridinyl)piperazine-1-carboxylate (3 g, 82%) as a yellow solid.LCMS (ESI+): m/z: 342.57 [M+H]+.
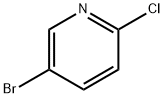
53939-30-3
483 suppliers
$5.00/1g

57260-71-6
734 suppliers
$5.00/5g

153747-97-8
96 suppliers
$7.00/250mg
Yield: 81.5%
Reaction Conditions:
with potassium carbonate in acetonitrile at 110; for 12 h;
Steps:
17.1 Step 1’) Synthesis of tert-butyl 4-(5 -bromopyridin-2-yl)piperazine- 1 -carboxylate
General procedure: To 1,4-dioxane (30 mL) were added tert-butyl piperazine-1-carboxylate (2.89 g, 15.51 mmol), 5-bromo-2-chloropyrimidine (2.00 g, 10.34 mmol) and potassium carbonate (2.86 g, 20.68 mmol) sequentially. The mixture was heated to 110 °C, after stirring for 12 hours, the reaction mixture was cooled to room temperature, filtered and concentrated in vacuo. The residue was purified by silica gel chromatography (PE/EtOAc (v/v) = 20/1) to give the title compound as a pale yellow solid (3.15 g, 88.7%).; The title compound was prepared using tert-butyl piperazine-1-carboxylate (1.45 g, 7.79 mmol),5-bromo-2-chloropyridine (1.00 g, 5.20 mmol) and potassium carbonate (1.44 g, 10.39 mmol) in acetonitrile (30 mL) according to the process described in Step 4 of Example 1, and the crude product was purified by silica gel chromatography (PE/EtOAc (v/v) = 20/1) to give the title compound as a pale yellow solid (1.45 g, 8 1.5%).MS (ESI, pos. ion) m/z: 342.1 [M+H] and‘H NMR (600 MHz, CDC13): (ppm) 8.17 (d, J 2.4 Hz, 1H), 7.52 (dd, J 9.0, 2.5 Hz, 1H), 6.52 (d, J 9.0 Hz, 1H), 3.55 - 3.49 (m, 4H), 3.49 - 3.45 (m, 4H), 1.46 (s, 9H).
References:
SUNSHINE LAKE PHARMA CO., LTD.;ZHANG, Yingjun;JIN, Chuanfei;LIANG, Haiping;YI, Chao;ZHANG, Ji WO2016/192657, 2016, A1 Location in patent:Page/Page column 65

624-28-2
719 suppliers
$5.00/5g

57260-71-6
734 suppliers
$5.00/5g

153747-97-8
96 suppliers
$7.00/250mg
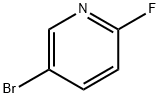
766-11-0
458 suppliers
$6.00/5g

57260-71-6
734 suppliers
$5.00/5g

153747-97-8
96 suppliers
$7.00/250mg

24424-99-5
859 suppliers
$13.50/25G
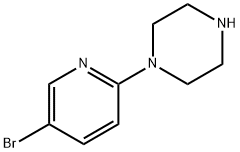
73406-97-0
72 suppliers
$48.00/1 / G

153747-97-8
96 suppliers
$7.00/250mg

624-28-2
719 suppliers
$5.00/5g
![8-BOC-3,8-DIAZA-BICYCLO[3.2.1]OCTANE](/CAS/GIF/149771-44-8.gif)
149771-44-8
216 suppliers
$14.00/250mg

153747-97-8
96 suppliers
$7.00/250mg