
4,6,7,8-TETRAHYDRO-1H,3H-QUINOLINE-2,5-DIONE synthesis
- Product Name:4,6,7,8-TETRAHYDRO-1H,3H-QUINOLINE-2,5-DIONE
- CAS Number:5057-12-5
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
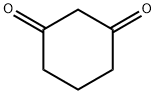
504-02-9
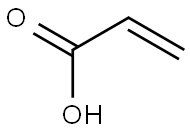
79-10-7

5057-12-5
General method: 1,3-Cyclohexanedione (8.9 mmol, 1 eq.) was mixed with acrylic acid (10.6 mmol, 1.2 eq.) in a reaction vial. Ammonium acetate (10.6 mmol, 1.2 eq.) was then added. The reaction mixture was thoroughly mixed using a spatula and heated with stirring at 80 °C for 5 h under nitrogen protection. After completion of the reaction, it was cooled to room temperature and the reaction mixture was diluted with 10 mL of distilled water followed by two extractions with ethyl acetate (2 × 10 mL). The organic extracts were combined and dried with anhydrous Na2SO4. After filtration, the solvent was removed by evaporation under reduced pressure and the resulting crude product was purified by column chromatography (eluent: ethyl acetate/hexane) to afford the target compound 3,4,7,8-tetrahydroquinoline-2,5(1H,6H)-dione. Characterization data of the compounds are provided.

504-02-9
582 suppliers
$5.00/10g

79-10-7
729 suppliers
$20.16/100 mL

5057-12-5
69 suppliers
$84.00/1g
Yield: 92%
Reaction Conditions:
with ammonium acetate at 80; for 5 h;Inert atmosphere;Michael Addition;Temperature;Time;Reagent/catalyst;
Steps:
General Procedure for Synthesis of 3,4,7,8-Tetrahydroquinoline-2,5(1H,6H)-dione (4a)
General procedure: 1,3-Dicarbonyls (8.9 mmol, 1 equiv.) followed by ammonium acetate (10.6 mmol, 1.2 mmol, 1.2 equiv.) were added to the solution of acrylic acid (10.6 mmol, 1.2 equiv.). The reactant mass was mixed thoroughly with a spatula and heated at 80 °C with stirring under a nitrogen atmosphere for 5 h. After cooling, the reaction mixture was diluted with 10mL distilled water and extracted twice with ethyl acetate (210 mL). The organic extracts were dried over anhydrous Na2SO4. After filtration, the solvent was evaporated under reduced pressure and subjected to column chromatography (ethyl acetate=hexane) to obtain pure products. The characterization data of the compounds are given.
References:
Senthil Kumar;Kumari, Neeta;Luthra, Pratibha Mehta [Synthetic Communications,2013,vol. 43,# 22,p. 3010 - 3019] Location in patent:supporting information

5220-49-5
232 suppliers
$13.19/1g

79-10-7
729 suppliers
$20.16/100 mL

5057-12-5
69 suppliers
$84.00/1g

504-02-9
582 suppliers
$5.00/10g

5057-12-5
69 suppliers
$84.00/1g