
3-CARBETHOXY-2-PIPERIDONE synthesis
- Product Name:3-CARBETHOXY-2-PIPERIDONE
- CAS Number:3731-16-6
- Molecular formula:C8H13NO3
- Molecular Weight:171.19

17216-62-5

3731-16-6
General procedure for the synthesis of ethyl 2-oxo-3-piperidine carboxylate from diethyl 2-cyanoethylmalonate: 241 kg of diethyl 2-cyanoethylmalonate, 1200 kg of isopropanol and 28 kg of Raney Co catalyst were added to a 2 L stainless steel autoclave. After replacing the air in the autoclave with hydrogen, the hydrogen pressure was adjusted to 25 kg/cm2, stirring was initiated, and the temperature was slowly raised to 70 °C by jacketed steam. Subsequently, oxygen was introduced to trigger the reaction exotherm, at which time cooling water was turned on to control the reaction temperature. The reaction temperature was maintained in the range of 90-100°C, and the hydrogen inlet was adjusted to stabilize the system pressure at 35-40 kg/cm2. Hydrogen supply was stopped after 4.5 h, and the reaction was continued at 100°C and 35 kg/cm2 for 1 h. After completion of the reaction, cooling water was used to cool the reaction. Upon completion of the reaction, the material in the kettle was cooled to 44°C with cooling water, the residual hydrogen pressure was released, and the material was transferred to a filter with nitrogen. The Raney Co catalyst was recovered by filtration under nitrogen protection. The filtrate was transferred to a 2000 L glass lined distillation crystallization kettle and distilled with heat and stirring to remove the isopropanol solvent. Subsequently, 660 kg of petroleum ether was added with continuous stirring for 0.5 hours. During slow cooling of the crystallization process, intermittent stirring was used to prevent agglomeration of the product and to facilitate subsequent drainage from the bottom of the kettle. After complete crystallization, the product was centrifuged and dried to give 184 kg of white solid product. The purity of the product was 99.6% by HPLC, and the yield of the hydrogenation reaction was 96.4%. The total yield of the two-step reaction was 79.2%.
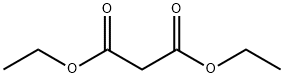
105-53-3
795 suppliers
$5.00/25g

3731-16-6
162 suppliers
$9.00/1g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: sodium ethylate / 65 °C / als Hauptprodukt
2: Raney nickel / 100 °C / 102971 Torr / Hydrogenation
References:
Koelsch [Journal of the American Chemical Society,1943,vol. 65,p. 2458]

17216-62-5
149 suppliers
$9.00/1g

3731-16-6
162 suppliers
$9.00/1g

2049-80-1
187 suppliers
$33.70/25g

3731-16-6
162 suppliers
$9.00/1g
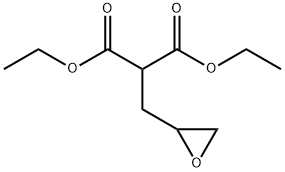
13353-23-6
3 suppliers
inquiry

3731-16-6
162 suppliers
$9.00/1g
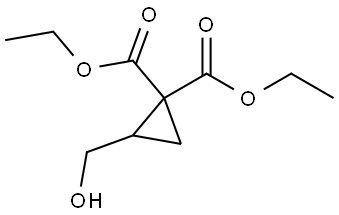
202336-20-7
0 suppliers
inquiry

3731-16-6
162 suppliers
$9.00/1g