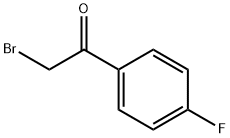
2-Bromo-4'-fluoroacetophenone synthesis
- Product Name:2-Bromo-4'-fluoroacetophenone
- CAS Number:403-29-2
- Molecular formula:C8H6BrFO
- Molecular Weight:217.04
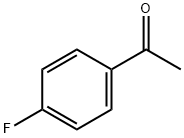
403-42-9

403-29-2
General procedure for the synthesis of 2-bromo-4'-fluoroacetophenone using 4-fluoroacetophenone as starting material: general method: oxone (1.352 g, 2.2 mmol) was added to a well-stirred methanol (10 ml) solution of 4-fluoroacetophenone (2 mmol) and NH4Br (0.215 g, 2.2 mmol), and the reaction mixture was stirred at room temperature (or refluxed at temperature reaction). The reaction progress was monitored by TLC. Upon completion of the reaction, the reaction mixture was quenched with aqueous sodium thiosulfate, followed by extraction with ethyl acetate (3 x 25 ml). The organic layers were combined, washed with water, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum to remove the solvent to give the crude product. The crude product was further purified by silica gel column chromatography (200-300 mesh) to give pure 2-bromo-4'-fluoroacetophenone. All products were structurally confirmed by 1H NMR and mass spectrometry data.

403-42-9
549 suppliers
$5.00/10g

403-29-2
326 suppliers
$8.00/5g
Yield:403-29-2 97%
Reaction Conditions:
with Oxone;ammonium bromide in methanol for 1.3 h;Reflux;regioselective reaction;
Steps:
2. General procedure
General procedure: Oxone (1.352 g, 2.2 mmol) was added to the well stirred solution of substrate (2 mmol) and NH4Br (0.215 g, 2.2 mmol) in methanol (10 ml) and the reaction mixture was allowed to stir at room temperature (or reflux temperature). After completion of the reaction, as monitored by TLC, the reaction mixture was quenched with aqueous sodium thiosulfate, and extracted with ethyl acetate (3×25 ml). Finally, the combined organic layer was washed with water, dried over anhydrous sodium sulfate, filtered and removal of solvent in vacuo yielded a crude residue, which was further purified by column chromatography over silica gel (finer than 200 mesh) to afford pure products. All the products were identified on the basis of 1H NMR and mass spectral data.
References:
MacHarla, Arun Kumar;Chozhiyath Nappunni, Rohitha;Marri, Mahender Reddy;Peraka, Swamy;Nama, Narender [Tetrahedron Letters,2012,vol. 53,# 2,p. 191 - 195] Location in patent:supporting information; experimental part

405-99-2
225 suppliers
$10.00/1g

403-29-2
326 suppliers
$8.00/5g

95895-33-3
3 suppliers
inquiry

403-29-2
326 suppliers
$8.00/5g
766-98-3
249 suppliers
$6.00/1g

403-29-2
326 suppliers
$8.00/5g
7542-64-5
30 suppliers
$29.30/1g

403-29-2
326 suppliers
$8.00/5g