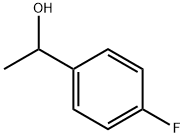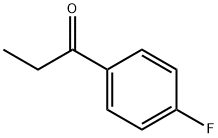
4'-Fluoropropiophenone synthesis
- Product Name:4'-Fluoropropiophenone
- CAS Number:456-03-1
- Molecular formula:C9H9FO
- Molecular Weight:152.17

5724-03-8

456-03-1
General procedure for the synthesis of 4'-fluoropropiophenone from 1-(4-fluorophenyl)prop-2-en-1-ol: Under nitrogen protection, the ruthenium complex [RuCl2(6-C6H6)(PTA-Me)] (3a) (0.02-0.1 mmol; 0.5-2.5 mol%) and K2CO3 (0.05-0.25 mmol) were added to a solution of 1-(4-fluorophenyl)prop-2-en-1-ol (4a-o, 4 mmol) in tetrahydrofuran (4mL). -fluorophenyl)prop-2-en-1-ol (4a-o, 4 mmol) in a solution of tetrahydrofuran (4 mL). The reaction mixture was placed in a sealed tube and stirred at 75°C for the indicated time (see Table 4 and Scheme 3). The progress of the reaction was monitored by sampling and diluting 10 μL of the sample with dichloromethane (3 mL) every ~1 hr followed by GC analysis. Upon completion of the reaction, the mixture was cooled to room temperature, at which point the ruthenium complex 3a partially precipitated. The solids were removed by filtration and the reaction products were subsequently separated by solvent evaporation. The residue was purified using silica gel column chromatography with EtOAc-hexane (1:10) mixed solvent as eluent. The structure of the resulting carbonyl compounds 5a-o was confirmed by GC/MS analysis of their fragmentation patterns, NMR spectroscopy, and comparison of retention times of commercially pure samples (Sigma-Aldrich or Acros Organics).
Yield:456-03-1 91%
Reaction Conditions:
with chlorine[2-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxypyridine](pentamethylcyclopentadienyl)iridium(III) chloride;potassium hydroxide in water at 80;Schlenk technique;Inert atmosphere;
Steps:
11
In a 10 mL Schlenk tube, methanol (1.1 mmol), 4-fluorophenethyl alcohol (1.0 mmol), KOH (1.1 mmol), H2O (2.0 mL) and TC-6 (0.1 mol%) were added. Under the protection of nitrogen, heat to 80°C for reaction. After completion, it was extracted with ethyl acetate (3×5.0 mL), the organic layers were combined, dried over anhydrous magnesium sulfate, and the solvent was concentrated under reduced pressure to obtain a crude product. The crude product was purified with a mixture of ethyl acetate/petroleum ether (1/50) as the eluent and purified by silica gel chromatography to obtain a pure product with a yield of 91%.
References:
CN112047797,2020,A Location in patent:Paragraph 0141-0148

104863-65-2
82 suppliers
$16.00/250mg

460-00-4
636 suppliers
$6.00/10g

456-03-1
223 suppliers
$6.00/5g

405-64-1
34 suppliers
$234.00/5g

456-03-1
223 suppliers
$6.00/5g