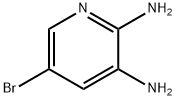
2,3-Diamino-5-bromopyridine synthesis
- Product Name:2,3-Diamino-5-bromopyridine
- CAS Number:38875-53-5
- Molecular formula:C5H6BrN3
- Molecular Weight:188.03

6945-68-2

38875-53-5
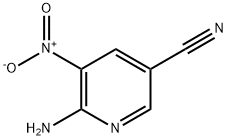
The general procedure for the synthesis of 2,3-diamino-5-bromopyridine from 2-amino-3-nitro-5-bromopyridine is as follows: Part I: Synthesis of imidazo[4,5-b]pyridin-2(3H)-one boronic acid Step 1: Preparation of 5-bromopyridine-2,3-diamine 5-Bromo-3-nitropyridin-2-amine (3 g) was dissolved in a solvent mixture of isopropanol (56 mL) and water (28 mL). Ammonium chloride (1.47 g, 2 eq.) and iron powder (2.31 g, 3 eq.) were subsequently added. The reaction mixture was heated to 90°C and kept at this temperature for 45 minutes. Upon completion of the reaction, the mixture was cooled, diluted with ethyl acetate (EtOAc), filtered to remove insoluble matter, and the organic and aqueous layers were separated. The organic layer was subsequently washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford the target product 2,3-diamino-5-bromopyridine as a solid (2.45 g, 95% yield). Product characterization data: 1H-NMR (300 MHz, DMSO-d6) δ 7.25 (d, 1H), 6.77 (d, 1H), 5.70-5.40 (bs, 2H), 5.20-4.80 (bs, 2H).

6945-68-2
407 suppliers
$9.00/5g

38875-53-5
354 suppliers
$6.00/1g
Yield:38875-53-5 95%
Reaction Conditions:
with aluminum (III) chloride;water;iron in isopropyl alcohol at 90; for 0.75 h;
Steps:
2.I.1
EXAMPLE 2; Representative Procedures for the Synthesis of a 1,4-Benzodiazepinone bearing a C5-1H- Imidazo[4,5-b]pyridin-2(3H)-one Group; .Part I: Synthesis of Imidazo[4,5-b]pyridin-2(3H)-one Boronic Acid; Step 1 5-Bromopyridine-2,3-diamine. 5-Bromo-3-nitropyridin-2-amine (3 g) was dissolved in isopropyl alcohol (56 mL) and water (28 mL). Ammonium chloride (1.47 g, 2 eq) was added followed by iron powder (2.31 g, 3 eq). The reaction was heated to 90 0C for 45 minutes. The solution was then cooled, and diluted with EtOAc, filtered, and the layers were separated. The organic layer was then washed with brine, dried over sodium sulfate, and concentrated delivering product as a solid (2.45 g, 95% yield). 1H-NMR (300 MHz, DMSO-d6) δ 7.25 (d, IH), 6.77 (d, IH), 5.70 - 5.40 (bs, 2H), 5.20 - 4.80 (bs, 2H).
References:
WO2010/121164,2010,A2 Location in patent:Page/Page column 60

6945-68-2
407 suppliers
$9.00/5g
7440-44-0
349 suppliers
$12.00/25g

38875-53-5
354 suppliers
$6.00/1g
1003711-13-4
12 suppliers
inquiry

38875-53-5
354 suppliers
$6.00/1g