
3-Amino-5-bromopyridine synthesis
- Product Name:3-Amino-5-bromopyridine
- CAS Number:13535-01-8
- Molecular formula:C5H5BrN2
- Molecular Weight:173.01

28733-43-9

13535-01-8
General procedure for the synthesis of 5-bromo-3-aminopyridine from 5-bromonicotinamide: 1. To a pre-cooled 340 ml aqueous solution containing 31.8 g (0.79 mol) of sodium hydroxide and 40.7 g (0.255 mol) of bromine, 42.0 g (0.209 mol) of commercially available 5-bromonicotinamide was added. 2. The reaction mixture was gradually warmed to room temperature, followed by heating the reaction at 70 °C for 1 hour. 3. Upon completion of the reaction, the resulting brown suspension was allowed to cool to room temperature. 4. The aqueous phase was treated with saturated brine and then extracted three times with a 1:1 solvent mixture of THF and tert-butyl methyl ether. 5. The organic phases were combined, dried with magnesium sulfate, filtered and concentrated under reduced pressure. 6. 39.1 g of the dark brown residue obtained from the concentration was purified by fast chromatography (eluent: heptane/ethyl acetate 1:1) to yield 5-bromo-3-aminopyridine as a brown solid (total yield 70.2 g, 70% yield).
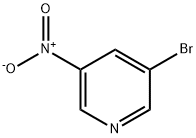
15862-30-3
142 suppliers
$11.00/250mg

13535-01-8
390 suppliers
$3.00/1g
Yield:13535-01-8 96%
Reaction Conditions:
with tetrahydroxydiboron;palladium on activated charcoal;water in acetonitrile at 50; for 24 h;Inert atmosphere;
Steps:
32 Example 32Synthesis of 5-bromo-3-aminopyridine
5-Bromo-3-nitropyridine (0.6mmol, 121.8mg), water (6mmol, 108.0mg), Pd/C (0.03mmol, 6.4mg) and tetrahydroxydiboron (1.98mmol, 177.5mg), acetonitrile ( 1mL), reacted at 50 C for 24h under nitrogen protection, monitored the reaction by TLC, added 10mL water, extracted with ethyl acetate (10mL×3), combined the organic phases, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated by column chromatography ( V petroleum ether: V ethyl acetate=3:1) to obtain 99.7 mg of a white solid, that is, the target compound is obtained with a yield of 96%.
References:
Yichang Shang Nuode Bio-pharmaceutical Technology Co., Ltd.;Zhou Haifeng;Zhou Yanmei;Pi Danwei;Liu Qixing;Liu Sensheng CN106800493, 2020, B Location in patent:Paragraph 0185-0188

28733-43-9
211 suppliers
$7.00/1g

13535-01-8
390 suppliers
$3.00/1g
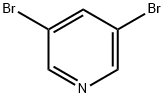
625-92-3
499 suppliers
$6.00/10g

13535-01-8
390 suppliers
$3.00/1g
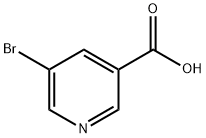
20826-04-4
561 suppliers
$5.00/1g

13535-01-8
390 suppliers
$3.00/1g
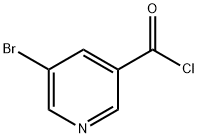
39620-02-5
79 suppliers
$82.00/10g

13535-01-8
390 suppliers
$3.00/1g