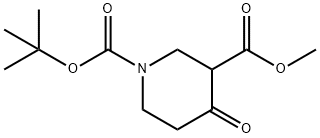
1-tert-Butyl 3-methyl 4-oxopiperidine-1,3-dicarboxylate synthesis
- Product Name:1-tert-Butyl 3-methyl 4-oxopiperidine-1,3-dicarboxylate
- CAS Number:161491-24-3
- Molecular formula:C12H19NO5
- Molecular Weight:257.28
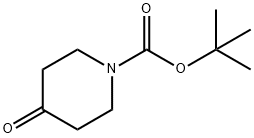
79099-07-3
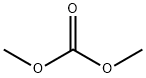
616-38-6

161491-24-3
60% sodium hydride (3.5 g, 88 mmol) was suspended in anhydrous toluene (50 mL) at room temperature and protected by nitrogen. Dimethyl carbonate (4.32 g, 48.0 mmol) was added slowly and dropwise to this suspension over a period of 0.5 hours. After the addition of a few drops of methanol, a solution of N-tert-butoxycarbonyl-4-piperidone (4.8 g, 24 mmol) dissolved in anhydrous toluene (20 mL) was added dropwise to the reaction mixture while maintaining the reaction system at 80 °C with stirring. The reaction mixture was continued to be stirred at the same temperature for 3 h. The reaction mixture was subsequently cooled to 0 °C (ice bath) and the pH was adjusted to 6-6.5 with acetic acid. the resulting cold mixture was diluted with water (10 mL) and the pH was adjusted to 8 with 5% sodium hydroxide solution. the toluene layer was separated and the aqueous layer was extracted with toluene (20 mL). The combined organic layers were dried with anhydrous sodium sulfate and concentrated under reduced pressure. The product was dried under vacuum to afford methyl methyl-1-tert-butoxycarbonyl-4-oxo-3-piperidinecarboxylate (5.0 g, 80% yield). The resulting compound did not require further purification and could be used directly in the subsequent reaction.

24424-99-5
865 suppliers
$13.50/25G
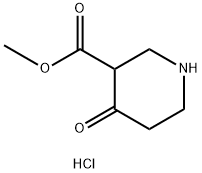
71486-53-8
145 suppliers
$6.00/1g

161491-24-3
175 suppliers
$8.00/1g
Yield:161491-24-3 100%
Reaction Conditions:
with sodium carbonate in tetrahydrofuran;water at 0; for 1 h;
Steps:
15i.1
Methyl 4-oxopiperidine-3-carboxylate hydrochloride (10.0 g, 51.6 mmol, 1 eq.) was dissolved in water (60 mL) at room temperature then cooled to 0° C. Added sodium carbonate (6.02 g, 56.8 mmol, 1.05 eq.) followed by the dropwise addition of BOC anhydride (11.84 g, 51.6 mmol, 1 eq.) in THF (50 mL) via an addition funnel. Stirred at 0° C. for 1 hour. Worked up by extracting 3 times with diethyl ether (50 mL). The diethyl ether extracts were combined and rinsed once (50 mL) with brine. The diethyl ether layer was dried over sodium sulfate and stripped to give 1-tert-butyl 3-methyl 4-oxopiperidine-1,3-dicarboxylate (13.29 g) as an amber oil. Yield=100%. 1H NMR (400 MHz) (CDCl3) δ 4.02 (s, 2H); 3.77 (s, 3H); 3.59 (s, 2H); 2.37 (s, 2H); 1.47 (s, 9H).
References:
US2005/54627,2005,A1 Location in patent:Page/Page column 114

24424-99-5
865 suppliers
$13.50/25G
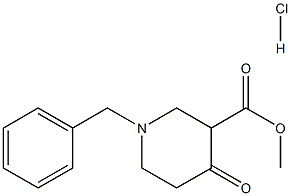
3939-01-3
131 suppliers
$5.00/1g

161491-24-3
175 suppliers
$8.00/1g

24424-99-5
865 suppliers
$13.50/25G

161491-24-3
175 suppliers
$8.00/1g

24424-99-5
865 suppliers
$13.50/25G

108554-34-3
52 suppliers
inquiry

161491-24-3
175 suppliers
$8.00/1g