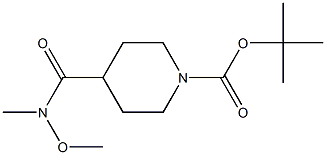
1-Boc-4-[methoxy(methyl)carbamoyl]piperidine synthesis
- Product Name:1-Boc-4-[methoxy(methyl)carbamoyl]piperidine
- CAS Number:139290-70-3
- Molecular formula:C13H24N2O4
- Molecular Weight:272.34

6638-79-5
84358-13-4
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
The general procedure for the preparation of 1-Boc-4-[methoxy(methyl)carbamoyl]piperidine from 1-Boc-4-piperidinecarboxylic acid (2.34 g, 10.2 mmol) and dimethylhydroxylamine hydrochloride (1.5 g, 15 mmol) in DMF (50 mL) was as follows: to a stirred 1-Boc-4-piperidinecarboxylic acid and dimethylhydroxylamine hydrochloride at room temperature in a suspension in DMF was added triethylamine (2.8 mL, 20 mmol). After stirring for 10 min, HOBt (1.62 g, 12 mmol) and EDCI (2.3 g, 12 mmol) were added sequentially. The reaction mixture was stirred overnight and subsequently concentrated. The concentrated residue was dissolved in 1N HCl (100 mL) and extracted with EtOAc (3 × 100 mL). The organic phases were combined, washed sequentially with saturated NaHCO3 solution (50 mL), brine (50 mL), dried over MgSO4 and concentrated to give a colorless oily product (2.79 g, yield >100%). The product was characterized by 1H NMR (500 MHz, CDCl3) and MS (ESI+): 1H NMR δ 4.14 (m, 2H), 3.71 (s, 3H), 3.19 (s, 3H), 2.78 (m, 3H), 1.68 (m, 4H), 1.46 (s, 9H); MS (ESI+) m/z 217.72 (M+H+- Isobutene).

6638-79-5
593 suppliers
$6.00/25g

84358-13-4
382 suppliers
$5.00/5g
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
184 suppliers
$6.00/100mg
Yield: 100%
Reaction Conditions:
Stage #1:N,O-dimethylhydroxylamine*hydrochloride;N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid with triethylamine in DMF (N,N-dimethyl-formamide) at 20; for 0.166667 h;
Stage #2: with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in DMF (N,N-dimethyl-formamide)
Stage #3: with hydrogenchloride in water
Steps:
3
To a stirring suspension of t-Boc-isonipecotic acid (19) (2.34 g, 10.2 mmol) and the Weinreb amine (1.5 g, 15 mmol) in DMF (50 mL), was added triethylamine (2.8 mL, 20 mmol) at room temperature. After stirring for 10 min, HOBt (1.62 g, 12 mmol) was added, followed by EDCI (2.3 g, 12 mmol). The resulting solution was stirred overnight and concentrated. The residue was taken up in 1 N HCl (100 mL) and extracted with EtOAc (3×100 mL). The combined organic extracts were washed with sat NaHCO3 (50 mL), brine (50 mL), dried (MgSO4) and concentrated to obtain a colorless oil (20) (2.79 g, >100%). 1H NMR (500 MHz, CDCL3) δ4.14 (m, 2H), 3.71 (s, 3H), 3.19 (s, 3H), 2.78 (m, 3H), 1.68 (m, 4H), 1.46 (s, 9H); MS (ESI+) m/z 217.72 (M+H+-isobutylene).
References:
Myriad Pharmaceuticals, Inc. US2004/14763, 2004, A1 Location in patent:Page 21

84358-13-4
382 suppliers
$5.00/5g
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
184 suppliers
$6.00/100mg

6638-79-5
593 suppliers
$6.00/25g
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
184 suppliers
$6.00/100mg

84358-13-4
382 suppliers
$5.00/5g

1117-97-1
71 suppliers
inquiry
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
184 suppliers
$6.00/100mg

142851-03-4
285 suppliers
$5.00/1g

1117-97-1
71 suppliers
inquiry
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
184 suppliers
$6.00/100mg