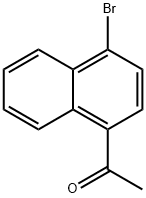
1-(1-BROMONAPHTHALEN-4-YL)ETHANONE synthesis
- Product Name:1-(1-BROMONAPHTHALEN-4-YL)ETHANONE
- CAS Number:46258-62-2
- Molecular formula:C12H9BrO
- Molecular Weight:249.1

90-11-9

75-36-5

46258-62-2
1. 1-Bromonaphthalene (10 g, 48.3 mmol) and acetyl chloride (4.2 ml, 58 mmol) were dissolved in 1,2-dichloroethane (100 ml) at 0 °C and aluminum chloride (14.4 g, 108 mmol) was added in batches. 2. The reaction mixture was stirred at room temperature for 24 hours. 3. The reaction mixture was poured into ice water (100 ml) and the organic and aqueous layers were separated. 4. The aqueous layer was extracted with ether (3 x 150 ml), the organic layers were combined and dried with magnesium sulfate. 5. After filtration, the solvent was removed under reduced pressure to give an orange colored oil. 6. Purification by fast chromatography (cyclohexane/ethyl acetate: 95/5) afforded 1-(4-bromo-1-naphthyl)ethanone as a yellow oil (91% yield). 7. 1-(4-bromo-1-naphthyl)ethanone was converted to its oxime derivative according to a method similar to that used for the preparation of compound 22 to afford a white powder (98% yield). 8. Activated zinc powder (24.7 g, 379 mmol) was added batchwise to a suspension of oxime (10.0 g, 37.9 mmol) in acetic acid (40 ml) and stirred at room temperature for 2 hours. 9. Zinc powder was removed by filtration and acetic acid was removed under reduced pressure. 10. Water (100 ml) was added and the pH was adjusted to 13 with 1N NaOH. 11. The aqueous layer was extracted with EtOAc (3 x 100 ml), the organic layers were combined and dried with MgSO4. 12. After filtration, the solvent was removed under reduced pressure to give a yellow oil (70% yield). 13. Boc2O (7.1 g, 31.8 mmol) was added to a 1,4-dioxane (50 ml) solution of amine (6.6 g, 26.5 mmol) and stirred at room temperature for 2 hours. 14. The solvent was removed under pressure and purified by fast chromatography (cyclohexane/EtOAc: 95/5) to give a yellow powder (75% yield). 15. Bromide (350 mg, 1 mmol) was dissolved in THF (13 ml)/water (2 ml), potassium acetate (100 mg, 1 mmol), 1,3-bis(diphenylphosphino)propane (9.0 mg, 0.02 mmol) and palladium(II) acetate (9.0 mg, 0.04 mmol) were added. 16. stirring for 3 hours at 50 atm CO pressure and 150 °C. 17. The reaction mixture was filtered, the filtrate was dried over MgSO4 and the solvent was removed under reduced pressure to give a yellow-green oil (300 mg). 18. Purification by fast chromatography (DCM/MeOH: 90/10) afforded 4-(1-tert-butoxycarbonylaminoethyl)-naphthalene-1-carboxylic acid as a white powder (14% yield). 19. The title product was prepared according to the procedure for compound 31 from 4-(1-tert-butoxycarbonylaminoethyl)-naphthalene-1-carboxylic acid (44 mg) and 4-aminopyridine (67% yield). NMR data (300 MHz, DMSO-d6): δ 1.64 (d, 3H, J=6.6 Hz); 5.3 (q, 1H, J=6.5 Hz); 7.71 (m, 1H); 8.00 (d, 1H, J=7.7 Hz); 8.32 (m, 1H); 8.35 (d, 1H, J=7.3 Hz); 8.81 (d, 2H J=7.2Hz); 12.2 (s, 1H).

90-11-9
542 suppliers
$5.00/10g

75-36-5
592 suppliers
$17.92/100G

46258-62-2
99 suppliers
$24.00/100mg
Yield: 91%
Reaction Conditions:
with aluminum (III) chloride in 1,2-dichloro-ethane at 0 - 20; for 24 h;
Steps:
3
A solution of 1-Bromo-naphthalene (10 g, 48.3 mmol) and acetyl chloride (4.2 ml, 58 mmol) in 1,2-dichloroethane (100 ml) was cooled to 0°C and aluminum chloride (14.4 g, 108 mmol) was added portion wise. The mixture was stirred at RT for 24 hours. The reaction mixture was poured into ice-water (100 ml). The two layers were separated and the water layer was extracted with diethyl ether (3 x 150 ml). The combined organic layers were dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure to give an orange colored oil. The 1- (4-bromo-naphtalen-1-yl)-ethanone was purified by flash chromatography (cyclohexane/ethylacetate: 95/5), yielding an yellow oil (91% yield). The 1- (4-bromo-naphtalen-1-yl)-ethanone oxime was prepared according to the procedure described for Compound 22, yielding a white powder (98% yield). Activated zinc dust (24.7 g, 379 mmol) was added portion wise to a suspension of the oxime (10.0 g, 37.9 mmol) in acetic acid (40 ml). The mixture was stirred at RT for 2 hours. The zinc dust was removed by filtration and acetic acid was removed under reduced pressure. Water (100 ml) was added and the pH was adjusted to pH = 13 with 1N NaOH. The water layer was extracted with EtOAc (3 x 100 ml). The combined organic layers were dried over MgS04, filtered and the solvent was removed under reduced pressure, yielding a yellow oil (70% yield). Boc20 (7.1 g, 31.8 mmol) was added to a solution of the amine (6.6 g, 26.5 mmol) in 1,4-dioxane (50 ml). The reaction mixture was stirred at RT for 2 hours. The solvent was removed under reduced pressure and the product was purified by flash chromatography (cyclohexane/EtOAc: 95/5), yielding a yellow powder (75% yield). The bromide (350 mg, 1 mmol) was dissolved in THF (13 ml) /water (2 ml). Potassium acetate (100 mg, 1 mmol), 1,3-bis-diphenylphosphinopropane (9.0 mg, 0.02 mmol) and palladium- (11)-acetate (9.0 mg, 0.04 mmol) were added. The mixture was stirred at 50 atm CO pressure and 150°C for 3 hours. The reaction mixture was filtered, the filtrate dried over MgS04 and the solvent was removed under reduced pressure to give a yellow- greenish oil (300 mg). The 4- (l-tert-butoxycarbonylamino-ethyl)-naphthalene-l- carboxylic acid was purified by flash chromatography (DCM/MeOH : 90/10), yielding a white powder (14% yield). The title product was prepared according to the procedure of Compound 31, starting from 4- (1-tert-butoxycarbonylamino-ethyl)-naphthalene-1-carboxylic acid (44 mg) and 4-amino-pyridine (67% yield).'H NMR (300 MHz, , DMSO-d6): 1.64 ppm (d, 3H, J = 6.6 Hz); 5.3 ppm (q, 1H, J = 6.5 Hz), 7.71 ppm (m, 1H), 8.00 ppm (d, 1H, J = 7.7 Hz), 8.32 ppm (m, 1H), 8. 35 ppm (d, 1H, J = 7.3 Hz), 8.81 ppm (d, 2H, J = 7.2 Hz), 12.2 ppm (s, 1H).
References:
DEVGEN NV WO2005/82367, 2005, A1 Location in patent:Page/Page column 64-65

58149-70-5
1 suppliers
inquiry

46258-62-2
99 suppliers
$24.00/100mg
83-53-4
344 suppliers
$10.00/1g

97674-02-7
235 suppliers
$15.00/1 g

46258-62-2
99 suppliers
$24.00/100mg

90-11-9
542 suppliers
$5.00/10g
108-24-7
0 suppliers
$14.00/250ML

46258-62-2
99 suppliers
$24.00/100mg

75-15-0
267 suppliers
$40.00/100 g
7446-70-0
713 suppliers
$15.00/25g

90-11-9
542 suppliers
$5.00/10g

75-36-5
592 suppliers
$17.92/100G

46258-62-2
99 suppliers
$24.00/100mg